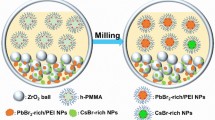
Abstract
To enhance the stability in humidity is very crucial to hybrid organic-inorganic lead halide perovskites in a broad range of applications. This report describes a coating stratergy of perovskite nanocrystals via polymethylmethacrylate-introduced ligand-assisted reprecipitation, using the interactions between the Pb cations on the surface of perovskite nanocrystals and the functional ester carbonyl groups in polymethylmethacrylate framework. The hydrophobic framework shields the open metal sites of hybrid organic-inorganic lead halide perovskites from being attacked by water, effectively retarding the diffusion of water into the perovskite nanocrystals. The as-prepared films demonstrate high resistance to heat and moisture. Additionally, the introduction of polymethylmethacrylate into ligand-assisted reprecipitation can effectively control the bulk precipitation and promote the stability of the perovskite solution.
摘要
有机-无机杂化铅卤钙钛矿易于加工、 带隙可调、 电荷转移速率高, 是一种具有广泛应用前景的新型光电半导体材料. 在潮湿空气中的稳定性是钙钛矿实现产业化应用亟待解决的问题. 本文介绍了聚甲基丙烯酸甲酯作为配体利用配体辅助再沉淀实现了钙钛矿纳米晶的聚合物包裹.聚合物作为疏水性骨架通过功能性酯羰基与钙钛矿表面铅化学键合实现了表面铅位点的全覆盖, 有效阻止该位点被水分子占据, 形成的紧密界面层有效延缓水分子扩散到钙钛矿纳米晶中. 制备的薄膜表现出超高的浸水稳定性.
Similar content being viewed by others
References
Kojima A, Teshima K, Shirai Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc, 2009, 131: 6050–6051
Lee MM, Teuscher J, Miyasaka T, et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science, 2012, 338: 643–647
Yin X, Xu Z, Guo Y, et al. Ternary oxides in the TiO2-ZnO system as efficient electron-transport layers for perovskite solar cells with efficiency over 15%. ACS Appl Mater Interfaces, 2016, 8: 29580–29587
Yin X, Guo Y, Xue Z, et al. Performance enhancement of perovskite-sensitized mesoscopic solar cells using Nb-doped TiO2 compact layer. Nano Res, 2015, 8: 1997–2003
Cho H, Jeong SH, Park MH, et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science, 2015, 350: 1222–1225
Tan ZK, Moghaddam RS, Lai ML, et al. Bright light-emitting diodes based on organometal halide perovskite. Nat Nanotech, 2014, 9: 687–692
Qin X, Dong H, Hu W. Green light-emitting diode from bromine based organic-inorganic halide perovskite. Sci China Mater, 2015, 58: 186–191
Zhu H, Fu Y, Meng F, et al. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat Mater, 2015, 14: 636–642
Dou L, Yang YM, You J, et al. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat Commun, 2014, 5: 5404
Xue M, Zhou H, Xu Y, et al. High-performance ultraviolet-visible tunable perovskite photodetector based on solar cell structure. Sci China Mater, 2017, 60: 407–414
Leijtens T, Eperon GE, Noel NK, et al. Stability of metal halide perovskite solar cells. Adv Energy Mater, 2015, 5: 1500963
Rong Y, Liu L, Mei A, et al. Beyond efficiency: the challenge of stability in mesoscopic perovskite solar cells. Adv Energy Mater, 2015, 5: 1501066
Berhe TA, Su WN, Chen CH, et al. Organometal halide perovskite solar cells: degradation and stability. Energy Environ Sci, 2016, 9: 323–356
Wei J, Shi C, Zhao Y, et al. Potentials and challenges towards application of perovskite solar cells. Sci China Mater, 2016, 59: 769–778
Mosconi E, Azpiroz JM, De Angelis F. Ab initio molecular dynamics simulations of methylammonium lead iodide perovskite degradation by water. Chem Mater, 2015, 27: 4885–4892
Haruyama J, Sodeyama K, Han L, et al. Termination dependence of tetragonal CH3NH3PbI3 surfaces for perovskite solar cells. J Phys Chem Lett, 2014, 5: 2903–2909
Li B, Fei C, Zheng K, et al. Constructing water-resistant CH3NH3-PbI3 perovskite films via coordination interaction. J Mater Chem A, 2016, 4: 17018–17024
Li X, Ibrahim Dar M, Yi C, et al. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω-ammonium chlorides. Nat Chem, 2015, 7: 703–711
Guarnera S, Abate A, Zhang W, et al. Improving the long-term stability of perovskite solar cells with a porous Al2O3 buffer layer. J Phys Chem Lett, 2015, 6: 432–437
Conings B, Drijkoningen J, Gauquelin N, et al. Intrinsic thermal instability of methylammonium lead trihalide perovskite. Adv Energy Mater, 2015, 5: 1500477
Habisreutinger SN, Leijtens T, Eperon GE, et al. Carbon nanotube/polymer composites as a highly stable hole collection layer in perovskite solar cells. Nano Lett, 2014, 14: 5561–5568
Huang S, Li Z, Kong L, et al. Enhancing the stability of CH3NH3-PbBr3 quantum dots by embedding in silica spheres derived from tetramethyl orthosilicate in “waterless” toluene. J Am Chem Soc, 2016, 138: 5749–5752
Di D, Musselman KP, Li G, et al. Size-dependent photon emission from organometal halide perovskite nanocrystals embedded in an organic matrix. J Phys Chem Lett, 2015, 6: 446–450
Zhou Q, Bai Z, Lu WG, et al. In situ fabrication of halide perovskite nanocrystal-embedded polymer composite films with enhanced photoluminescence for display backlights. Adv Mater, 2016, 28: 9163–9168
Wang Y, He J, Chen H, et al. Ultrastable, highly luminescent organic-inorganic perovskite-polymer composite films. Adv Mater, 2016, 28: 10710–10717
Raja SN, Bekenstein Y, Koc MA, et al. Encapsulation of perovskite nanocrystals into macroscale polymer matrices: enhanced stability and polarization. ACS Appl Mater Interfaces, 2016, 8: 35523–35533
Chen K, Schünemann S, Tüysüz H. Preparation of waterproof organometal halide perovskite photonic crystal beads. Angew Chem Int Ed, 2017, 56: 6548–6552
Tannenbaum R, Zubris M, David K, et al. FTIR characterization of the reactive interface of cobalt oxide nanoparticles embedded in polymeric matrices. J Phys Chem B, 2006, 110: 2227–2232
Huang H, Susha AS, Kershaw SV, et al. Control of emission color of high quantum yield CH3NH3PbBr3 perovskite quantum dots by precipitation temperature. Adv Sci, 2015, 2: 1500194
Zhang F, Zhong H, Chen C, et al. Brightly luminescent and colortunable colloidal CH3NH3PbX3 (X=Br, I, Cl) quantum dots: potential alternatives for display technology. ACS Nano, 2015, 9: 4533–4542
Tannenbaum R, King S, Lecy J, et al. Infrared study of the kinetics and mechanism of adsorption of acrylic polymers on alumina surfaces. Langmuir, 2004, 20: 4507–4514
Ciprari D, Jacob K, Tannenbaum R. Characterization of polymer nanocomposite interphase and its impact on mechanical properties. Macromolecules, 2006, 39: 6565–6573
Zhu F, Men L, Guo Y, et al. Shape evolution and single particle luminescence of organometal halide perovskite nanocrystals. ACS Nano, 2015, 9: 2948–2959
Konstadinidis K, Thakkar B, Chakraborty A, et al. Segment level chemistry and chain conformation in the reactive adsorption of poly(methyl methacrylate) on aluminum oxide surfaces. Langmuir, 1992, 8: 1307–1317
Zeng R, Rong MZ, Zhang MQ, et al. Interfacial interaction in Ag/polymer nanocomposite films. J Mater Sci Lett, 2001, 20: 1473–1476
Li X, Wu Y, Zhang S, et al. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv Funct Mater, 2016, 26: 2435–2445
de Quilettes DW, Vorpahl SM, Stranks SD, et al. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science, 2015, 348: 683–686
Zheng K, Zhu Q, Abdellah M, et al. Exciton binding energy and the nature of emissive states in organometal halide perovskites. J Phys Chem Lett, 2015, 6: 2969–2975
Schmidt T, Lischka K, Zulehner W. Excitation-power dependence of the near-band-edge photoluminescence of semiconductors. Phys Rev B, 1992, 45: 8989–8994
Dar MI, Jacopin G, Meloni S, et al. Origin of unusual bandgap shift and dual emission in organic-inorganic lead halide perovskites. Sci Adv, 2016, 2: e1601156
Shobhana E. X-ray diffraction and UV-visible studies of PMMA thin films. Int J Modern Eng Res, 2012, 2: 1092–1095
N’Diaye M, Pascaretti-Grizon F, Massin P, et al. Water absorption of poly(methyl methacrylate) measured by vertical interference microscopy. Langmuir, 2012, 28: 11609–11614
Acknowledgements
This work was supported by the Thousand Young Talents Program, and the National Natural Science Foundation of China (21422507, 21635002 and 21321003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiao Li is now a Master candidate in materials science and engineering at the North University of China. His current research interests are focused on the synthesis of perovskite nanomaterials and polymer composites.
Tie Wang is a professor at the Institute of Chemistry, Chinese Academy of Sciences (ICCAS). He received his PhD (2007) from Changchun Institute of Applied Chemistry. He joined the ICCAS with the award of the “Thousand Youth Talents Plan” in 2013. His research is focused on the assemblies and applications of nanoparticles.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, X., Xue, Z., Luo, D. et al. A stable lead halide perovskite nanocrystals protected by PMMA. Sci. China Mater. 61, 363–370 (2018). https://doi.org/10.1007/s40843-017-9148-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-017-9148-7