Abstract
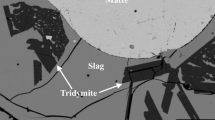
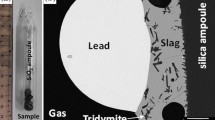
To assist in improving the recoveries of gold and silver in both primary production and metals’ recycling, the distributions of Ag and Au between slag, matte, and metal in the Cu–Fe–O–S–Si system have been investigated using an integrated experimental and thermodynamic modeling approach. The experimental technique involved high-temperature equilibration at selected temperatures and gas atmospheres, and rapid quenching followed up by measurement of phase compositions using microanalysis techniques. Electron probe X-ray microanalysis was used to measure the major element concentrations in the condensed phases. The laser ablation inductively coupled plasma mass spectrum technique was used to measure the concentrations of Ag and Au in slag. The experimentally determined distribution coefficient of Ag between matte and slag decreases with the increasing matte grade below approximately 70% Cu, but then increases with the increasing matte grade between 70 and 80% Cu. The experimentally determined distribution coefficient of Au between matte and slag decrease from log L = − 2.8 for approximately 63% Cu to approximately log L = − 3.8 at 76% Cu. Thermodynamic model parameters describing Ag and Au distributions have been derived following consideration of available data on slag/matte, slag/metal, and slag/matte/metal systems.




Similar content being viewed by others
References
Khaliq A, Rhamdhani MA, Brooks G, Masood S (2014) Metal extraction processes for electronic waste and existing industrial routes: a review and Australian perspective. Resources 3:152–179. https://doi.org/10.3390/resources3010152
Tesfaye F, Lindberg D, Hamuyuni J, Taskinen P, Hupa L (2017) Improving urban mining practices for optimal recovery of resources from e-waste. Miner Eng 111:209–221. https://doi.org/10.1016/j.mineng.2017.06.018
Forsén O, Aromaa J, Lundström M (2017) Primary copper smelter and refinery as a recycling plant—a system integrated approach to estimate secondary raw material tolerance. Recycling 2:19. https://doi.org/10.3390/recycling2040019
Schlesinger ME, King MJ, Sole KC, Davenport WGI (eds) (2011) Extractive metallurgy of copper. Elsevier, Amsterdam
Shishin D, Hayes PC, Jak E (2018) Multicomponent thermodynamic databases for complex non-ferrous pyrometallurgical processes. In: Davis B, Moats M, Wang S (eds) Extraction 2018, Ottawa, Canada. Springer, Berlin, pp 853–868
Shuva MAH, Rhamdhani MA, Brooks GA, Masood S, Reuter MA (2016) Thermodynamics data of valuable elements relevant to e-waste processing through primary and secondary copper production: a review J Clean. Prod 131:795–809. https://doi.org/10.1016/j.jclepro.2016.04.061
Avarmaa K, O’Brien H, Taskinen P (2016) Equilibria of gold and silver between molten coppe.–SiO2–Al2O3 slag in WEEE smelting at 1300 °C. In: Reddy RG, Pistorius PC, Pal U (eds) Advances in molten slags, fluxes, and salts: proceedings of the 10th international conference on molten slags, fluxes and salts (MOLTEN16). The Minerals, Metals & Materials Society, pp 193–202
Sukhomlinov D, Taskinen P (2017) Distribution of Ni, Co, Ag, Au, Pt, Pd between copper metal and silica saturated iron silicate slag. In: Proceedings of EMC 2017, Leipzig, Germany. pp 1–10
Hidayat T, Chen J, Hayes PC, Jak E (2018) Distributions of Ag, Bi, and Sb as minor elements between iron-silicate slag and copper in equilibrium with tridymite in the Cu–Fe–O–Si system at T = 1250 °C and 1300 °C (1523 K and 1573 K). Metall Mater Trans B. https://doi.org/10.1007/s11663-018-1448-8
Avarmaa K, O’Brien H, Johto H, Taskinen P (2015) Equilibrium distribution of precious metals between slag and copper matte at 1250–1350 °C. J Sustain Metall 1:216–228
Yamaguchi K (2010) Distribution of precious metals between matte and slag and precious metal solubility in slag. In: Proceedings of the Copper 2010, Hamburg, Germany, June 6–10, 2010. GDMB Informations GmbH, pp 1287–1295
Roghani G, Takeda Y, Itagaki K (2000) Phase equilibrium and minor element distribution between FeOx–SiO2–MgO-based slag and Cu2S–FeS matte at 1573 K under high partial pressures of SO2. Metall Mater Trans B 31B:705–712
Djordjevic P, Mitevska N, Mihajlovic I, Nikolic D, Manasijevic D, Zivkovic Z (2012) The effect of copper content in the matte on the distribution coefficients between the slag and the matte for certain elements in the sulphide copper concentrate smelting process J Min Metall. Sect B 48:143–151. https://doi.org/10.2298/jmmb111115012d
Jak E (2012) Integrated experimental and thermodynamic modelling research methodology for metallurgical slags with examples in the copper production field. Paper presented at the 9th international conference on molten slags, fluxes and salts (MOLTEN12), Beijing, China
Jak E, Hayes PC, Lee H-G (1995) Improved methodologies for the determination of high temperature phase equilibria Korean. J Miner Mater Inst 1:1–8
Fallah-Mehrjardi A, Hidayat T, Hayes PC, Jak E (2017) Experimental investigation of gas/slag/matte/tridymite equilibria in the Cu–Fe–O–S–Si system in controlled gas atmospheres: development of technique. Metall Mater Trans B 48:3002–3016. https://doi.org/10.1007/s11663-017-1073-y
Bale CW et al (2016) FactSage thermochemical software and databases, 2010–2016. CALPHAD 54:35–53. https://doi.org/10.1016/j.calphad.2016.05.002
Chen J, Allen CM, Azekenov T, Ushkov LA, Hayes P, Jak E (2016) Quantitative determination of trace/ultra trace elements concentration in slag and matte generated in copper smelting using microanalysis techniques. In: Copper 2016, Kobe, Japan
Shishin D, Decterov SA (2012) Critical assessment and thermodynamic modeling of Cu–O and Cu–O–S systems CALPHAD 38:59–70
Chartrand P, Pelton AD (2001) The modified quasichemical model. III—two sublattices. Metall Mater Trans A 32:1397–1407
Pelton AD, Chartrand P, Eriksson G (2001) The modified quasichemical model. IV—two sublattice quadruplet approximation. Metall Mater Trans A 32:1409–1415
Pelton AD, Decterov SA, Eriksson G, Robelin C, Dessureault Y (2000) The modified quasichemical model. I—binary solutions. Metall Mater Trans B 31:651–659
Pelton AD, Chartrand P (2001) The modified quasichemical model. II—multicomponent solutions. Metall Mater Trans A 32:1355–1360
Hillert M, Jansson B, Sundman B (1988) Application of the compound-energy model to oxide systems. Z Metallkd 79:81–87
Hidayat T, Shishin D, Jak E, Decterov S (2015) Thermodynamic reevaluation of the Fe–O system. CALPHAD 48:131–144
Waldner P, Pelton AD (2005) Thermodynamic modeling of the Fe–S system. J Phase Equilib Diffus 26:23–28
Shishin D (2013) Development of a thermodynamic database for copper smelting and converting. Ph. D. thesis, Ecole Polytechnique of Montreal
Shishin D, Hidayat T, Jak E, Decterov S (2013) Critical assessment and thermodynamic modeling of Cu–Fe–O system. CALPHAD 41:160–179
Shishin D, Jak E, Decterov SA (2015) Critical Assessment and Thermodynamic Modeling of the Fe–O–S system. J Phase Equilib Diffus 36:224–240. https://doi.org/10.1007/s11669-015-0376-4
Shishin D, Jak E, Decterov SA (2015) Thermodynamic assessment and database for the Cu–Fe–O–S system. CALPHAD 50:144–160
Hidayat T, Shishin D, Decterov S, Jak E (2017) Critical assessment and thermodynamic modeling of the Cu–Fe–O–Si system. CALPHAD 58:101–114
Shishin D, Decterov SA, Jak E (2018) Thermodynamic assessment of slag–matte–metal equilibria in the Cu–Fe–O–S–Si system. J Phase Equilib Diffus 39:456–475
Hidayat T, Shishin D, Decterov SA, Jak E (2017) Experimental study and thermodynamic re-evaluation of the FeO–Fe2O3–SiO2 system. J Phase Equilib Diffus 38:477–492. https://doi.org/10.1007/s11669-017-0535-x
Eriksson G, Pelton AD (1993) Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CaO–Al2O3, Al2O3–SiO2, and CaO–Al2O3–SiO2 systems. Metall Trans 24:807–816
Waldner P, Pelton AD (2006) Thermodynamic modeling of the Cu–Fe–S system. Internal report, Montreal, QC, Canada
Fallah-Mehrjardi A, Hidayat T, Hayes PC, Jak E (2017) Experimental investigation of gas/slag/matte/tridymite equilibria in the Cu–Fe–O–S–Si system in controlled gas atmospheres: experimental results at T = 1473 K [1200°C] and P(SO2) = 0.25 atm. Metall Mater Trans B 48:3017–3026. https://doi.org/10.1007/s11663-017-1076-8
Chen J, Hayes PC, Jak E (2017) Distributions of Ag and Sb as minor elements between slag, matte and metal in equilibrium with tridymite in the Cu–Fe–O–S–Si system: experimental results at T=1200, 1250 and 1300 °C private communications. The University of Queensland, Pyrometallurgy Innovation Centre
Lambotte G (2012) Approche thermodynamique de la corrosion des réfractaires aluminosiliceux par le bain cryolithique: modélisation thermodynamique du système quaternaire réciproque AlF3–NaF–SiF4–Al2O3–Na2O–SiO2. Ph. D. thesis, Université de Montréal, École Polytechnique de Montréal
Sharma RC, Chang YA (1986) The silver-sulfur system. Bull Alloy Phase Diagr 7:263–269. https://doi.org/10.1007/bf02869003
Acknowledgements
The authors gratefully appreciate the financial and technical support for this research from the consortium of metallurgical companies: Altonorte Glencore, Atlantic Copper, Aurubis, BHP Billiton Olympic Dam Operation, Kazzinc Glencore, PASAR Glencore, Outotec Oy (Espoo), Anglo American Platinum, Umicore, Rio Tinto Kennecott, and Boliden through Australian Research Council Linkage program LP140100480. The authors acknowledge the contribution of the AMMRF at the Centre for Microscopy and Microanalysis at The University of Queensland. The authors value greatly the scientific and technical assistance of Dr Charlotte Allen at the Centre of Analytical Research Facilities at the Queensland University of Technology, Brisbane, Australia. The authors offer their thanks to Mr Hong Wee Kor for technical support in the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Hiromichi Takebe.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shishin, D., Hidayat, T., Chen, J. et al. Experimental Investigation and Thermodynamic Modeling of the Distributions of Ag and Au Between Slag, Matte, and Metal in the Cu–Fe–O–S–Si System. J. Sustain. Metall. 5, 240–249 (2019). https://doi.org/10.1007/s40831-019-00218-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00218-w