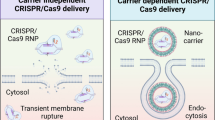
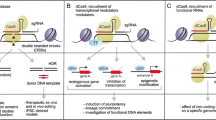
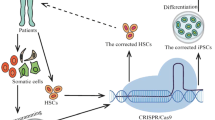
Abstract
Purpose of review
This review is focused on the recent development in the field of genomic editing of stem cells, in particular, the use of CRISPR/Cas9 systems to edit hemopoietic stem cells for beta-globinopathies and immunodeficiency syndromes. Additionally, the use of optimized genome-editing technologies in induced pluripotent stem cells will allow all affected tissue systems to be addressed.
Recent findings
The convergence of stem cell technologies and efficient genome-editing tools has brought forth the possibility of correcting human genetic diseases for the alleviation of human suffering. Meanwhile, germline editing of human zygotes is rapidly gathering pace, albeit with rising ethical and social/ moral concerns. There are remaining issues with off-target effects and cellular toxicity that needs to be addressed in full before its clinical implementation.
Summary
The era of genome-edited stem cell therapy with CRISPR/cas9 is now becoming a reality with clinical trials scheduled to start in the coming year.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. First paper to come up with CRISPR/Cas9, a low cost, efficient and fairly precise gene editing technology.
Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao S, et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell. 2013;13(6):659–62.
Zhou J, Shen B, Zhang W, Wang J, Yang J, Chen L, et al. One-step generation of different immunodeficient mice with multiple gene modifications by CRISPR/Cas9 mediated genome engineering. Int J Biochem Cell Biol. 2014;46:49–55.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23.
Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. Elife. 2013;2:e00471.
Dever DP, Bak RO, Reinisch A, Camarena J, Washington G, Nicolas CE, et al. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539(7629):384–9.
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13(6):653–8.
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154(2):442–51.
Pulecio J, Verma N, Mejía-Ramírez E, Huangfu D, Raya A. CRISPR/Cas9-based engineering of the epigenome. Cell Stem Cell. 2017;21(4):431–47.
Zalatan JG, Lee ME, Almeida R, Gilbert LA, Whitehead EH, la Russa M, et al. Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160(1–2):339–50.
Cassidy SB, Schwartz S. Prader-Willi and Angelman syndromes. Disorders of genomic imprinting. Medicine (Baltimore). 1998;77(2):140–51.
Kelly TK, De Carvalho DD, Jones PA. Epigenetic modifications as therapeutic targets. Nat Biotechnol. 2010;28(10):1069–78.
Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science. 2016;351(6271):400–3.
Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Rivera RMC, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351(6271):403–7.
Tabebordbar M, Zhu K, Cheng JKW, Chew WL, Widrick JJ, Yan WX, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351(6271):407–11.
Hacein-Bey-Abina S, le Deist F, Carlier F, Bouneaud C, Hue C, de Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346(16):1185–93.
Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–58.
Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341(6148):1233151.
Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–9.
Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467(7313):318–22.
Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–10.
• Cyranoski D. Chinese scientists to pioneer first human CRISPR trial. Nature. 2016;535(7613):476–7 First phase 1 trial involving CRISPR to repair T cells of patients with metastatic non-small-cell lung cancer.
DiGiusto DL, Cannon PM, Holmes MC, Li L, Rao A, Wang J, et al. Preclinical development and qualification of ZFN-mediated CCR5 disruption in human hematopoietic stem/progenitor cells. Mol Ther Methods Clin Dev. 2016;3:16067.
AG, C.T. CRISPR therapeutics submits first clinical trial application for a CRISPR gene-edited therapy, CTX001 in β-thalassemia. 2017 7th Dec 2017 [cited 2018 Feb 20]; Available from: https://globenewswire.com/news-release/2017/12/07/1247360/0/en/CRISPR-Therapeutics-Submits-First-Clinical-Trial-Application-for-a-CRISPR-Gene-Edited-Therapy-CTX001-in-%CE%B2-thalassemia.html.
Rodriguez C. CRISPR therapeutics and vertex pharmaceuticals are taking action to start a first clinical trial with CRISPR/Cas9 in Europe in 2018. 2017 [cited 2018 20 Feb]; Available from: https://labiotech.eu/crispr-therapeutics-clinical-trials/.
Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125(17):2605–13.
Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–29.
Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806.
•• Xie F, et al. Seamless gene correction of beta-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res. 2014;24(9):1526–33 A seminal paper that demonstrated that CRISPR/Cas9 can correct mutations in β-globin gene.
Piel FB. The present and future global burden of the inherited disorders of hemoglobin. Hematol Oncol Clin North Am. 2016;30(2):327–41.
Gaziev J, Sodani P, Lucarelli G. Hematopoietic stem cell transplantation in thalassemia. Bone Marrow Transplant. 2008;42(Suppl 1):S41.
Clement-De Boers A, et al. Final height and hormonal function after bone marrow transplantation in children. J Pediatr. 1996;129(4):544–50.
Sebastiano V, Maeder ML, Angstman JF, Haddad B, Khayter C, Yeo DT, et al. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells. 2011;29(11):1717–26.
Hoban MD, Cost GJ, Mendel MC, Romero Z, Kaufman ML, Joglekar AV, et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood. 2015;125(17):2597–604.
Hoban MD, Lumaquin D, Kuo CY, Romero Z, Long J, Ho M, et al. CRISPR/Cas9-mediated correction of the sickle mutation in human CD34+ cells. Mol Ther. 2016;24(9):1561–9.
DeWitt MA, et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med. 2016;8(360):360ra134.
Cai L, Bai H, Mahairaki V, Gao Y, He C, Wen Y, et al. A universal approach to correct various HBB gene mutations in human stem cells for gene therapy of Beta-thalassemia and sickle cell disease. Stem Cells Transl Med. 2018;7(1):87–97.
Worth AJ, Thrasher AJ. Current and emerging treatment options for Wiskott-Aldrich syndrome. Expert Rev Clin Immunol. 2015;11(9):1015–32.
Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis. 2015;60(8):1176–83.
De Ravin SS, et al. Targeted gene addition in human CD34(+) hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat Biotechnol. 2016;34(4):424–9.
De Ravin SS, et al. CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. Sci Transl Med. 2017;9:372.
Diez B, Genovese P, Roman-Rodriguez FJ, Alvarez L, Schiroli G, Ugalde L, et al. Therapeutic gene editing in CD34(+) hematopoietic progenitors from Fanconi anemia patients. EMBO Mol Med. 2017;9(11):1574–88.
Lombardo A, Genovese P, Beausejour CM, Colleoni S, Lee YL, Kim KA, et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol. 2007;25(11):1298–306.
Joglekar AV, Hollis RP, Kuftinec G, Senadheera S, Chan R, Kohn DB. Integrase-defective lentiviral vectors as a delivery platform for targeted modification of adenosine deaminase locus. Mol Ther. 2013;21(9):1705–17.
Luo Y, et al. Targeted inhibition of the miR-199a/214 cluster by CRISPR interference augments the tumor tropism of human induced pluripotent stem cell-derived neural stem cells under hypoxic condition. Stem Cells Int. 2016;2016:3598542.
Xia G, Gao Y, Jin S, Subramony SH, Terada N, Ranum LPW, et al. Genome modification leads to phenotype reversal in human myotonic dystrophy type 1 induced pluripotent stem cell-derived neural stem cells. Stem Cells. 2015;33(6):1829–38.
Xu X, Tay Y, Sim B, Yoon SI, Huang Y, Ooi J, et al. Reversal of phenotypic abnormalities by CRISPR/Cas9-mediated gene correction in Huntington disease patient-derived induced pluripotent stem cells. Stem Cell Reports. 2017;8(3):619–33.
Wang L, Yi F, Fu L, Yang J, Wang S, Wang Z, et al. CRISPR/Cas9-mediated targeted gene correction in amyotrophic lateral sclerosis patient iPSCs. Protein Cell. 2017;8(5):365–78.
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62.
Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–9.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76.
Jang YY, Ye Z. Gene correction in patient-specific iPSCs for therapy development and disease modeling. Hum Genet. 2016;135(9):1041–58.
Deng XY, Wang H, Wang T, Fang XT, Zou LL, Li ZY, et al. Non-viral methods for generating integration-free, induced pluripotent stem cells. Curr Stem Cell Res Ther. 2015;10(2):153–8.
Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–46.
Huai C, Jia C, Sun R, Xu P, Min T, Wang Q, et al. CRISPR/Cas9-mediated somatic and germline gene correction to restore hemostasis in hemophilia B mice. Hum Genet. 2017;136(7):875–83.
Group TH. Statement on genome editing technologies and human germline genetic modification. 2015 [cited 2018 24 February].
Bioethics N.C.o. Genome editing-an ethical review. 2016 27 Jul 2018; Available from: http://nuffieldbioethics.org/wp-content/uploads/Genome-editing-an-ethical-review.pdf.
Bioethics N.C.o. Genome editing and human reproduction. 2018 27 Jul 2018; Available from: http://nuffieldbioethics.org/wp-content/uploads/Genome-editing-and-human-reproduction-FINAL-website.pdf.
Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014;345(6201):1184–8.
Reddy P, Ocampo A, Suzuki K, Luo J, Bacman SR, Williams SL, et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell. 2015;161(3):459–69.
Zou Q, Wang X, Liu Y, Ouyang Z, Long H, Wei S, et al. Generation of gene-target dogs using CRISPR/Cas9 system. J Mol Cell Biol. 2015;7(6):580–3.
Shao Y, Guan Y, Wang L, Qiu Z, Liu M, Chen Y, et al. CRISPR/Cas-mediated genome editing in the rat via direct injection of one-cell embryos. Nat Protoc. 2014;9(10):2493–512.
Yoshimi K, Kaneko T, Voigt B, Mashimo T. Allele-specific genome editing and correction of disease-associated phenotypes in rats using the CRISPR-Cas platform. Nat Commun. 2014;5:4240.
Heo YT, Quan X, Xu YN, Baek S, Choi H, Kim NH, et al. CRISPR/Cas9 nuclease-mediated gene knock-in in bovine-induced pluripotent cells. Stem Cells Dev. 2015;24(3):393–402.
Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096.
Doudna JA, Sontheimer EJ. Methods in enzymology. The use of CRISPR/Cas9, ZFNs, and TALENs in generating site-specific genome alterations. Preface. Methods Enzymol. 2014;546:xix–xx.
Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156(4):836–43.
Liu H, Chen Y, Niu Y, Zhang K, Kang Y, Ge W, et al. TALEN-mediated gene mutagenesis in rhesus and cynomolgus monkeys. Cell Stem Cell. 2014;14(3):323–8.
•• Liang P, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6(5):363–72 First published work on CRISPR/Cas9 gene editing of human zygotes, with repaired embryos genetically mosaic and containing high levels of off effect targets.
Kang X, He W, Huang Y, Yu Q, Chen Y, Gao X, et al. Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing. J Assist Reprod Genet. 2016;33(5):581–8.
Tang L, Zeng Y, du H, Gong M, Peng J, Zhang B, et al. CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol Gen Genomics. 2017;292(3):525–33.
Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413–9.
Dieter Egli MZ, Kosicki M, Church G, Bradley A, Jasin M. Inter-homologue repair in fertilized human eggs? 2017 28 Aug 2017 [cited 2018 28 February]; Available from: https://www.biorxiv.org/content/early/2017/08/28/181255.
Adikusuma F, et al. Large deletions induced by Cas9 cleavage. Nature. 2018;560(7717):E8–9.
Ma H, et al. Ma et al. reply. Nature. 2018;560(7717):E10–23.
Vassena R, Heindryckx B, Peco R, Pennings G, Raya A, Sermon K, et al. Genome engineering through CRISPR/Cas9 technology in the human germline and pluripotent stem cells. Hum Reprod Update. 2016;22(4):411–9.
Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med. 2013;19(9):1111–3.
Gammage PA, Rorbach J, Vincent AI, Rebar EJ, Minczuk M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol Med. 2014;6(4):458–66.
Minczuk M, Papworth MA, Kolasinska P, Murphy MP, Klug A. Sequence-specific modification of mitochondrial DNA using a chimeric zinc finger methylase. Proc Natl Acad Sci U S A. 2006;103(52):19689–94.
Minczuk M, Papworth MA, Miller JC, Murphy MP, Klug A. Development of a single-chain, quasi-dimeric zinc-finger nuclease for the selective degradation of mutated human mitochondrial DNA. Nucleic Acids Res. 2008;36(12):3926–38.
Wang S, Yi F, Qu J. Eliminate mitochondrial diseases by gene editing in germ-line cells and embryos. Protein Cell. 2015;6(7):472–5.
Araki M, Ishii T. International regulatory landscape and integration of corrective genome editing into in vitro fertilization. Reprod Biol Endocrinol. 2014;12:108.
Friedmann T, Jonlin EC, King NMP, Torbett BE, Wivel NA, Kaneda Y, et al. ASGCT and JSGT joint position statement on human genomic editing. Mol Ther. 2015;23(8):1282.
Bauquis C. More than 8 million babies born from IVF since the world’s first in 1978. 2018, Available from: https://www.eshre.eu/ESHRE2018/Media/ESHRE-2018-Press-releases/De-Geyter.aspx. Accessed 14 Aug 2018.
Committee U.I.B Report of the IBC on Updating its reflection on the human genome and human rights. 2015, Available from: http://unesdoc.unesco.org/images/0023/002332/233258E.pdf. Accessed 29 Dec 2017.
Fogarty NME, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, et al. Genome editing reveals a role for OCT4 in human embryogenesis. Nature. 2017;550(7674):67–73.
Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018.
Savic N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016;168:15–21.
Liu P, Chen M, Liu Y, Qi LS, Ding S. CRISPR-based chromatin remodeling of the endogenous Oct4 or Sox2 locus enables reprogramming to pluripotency. In: Cell Stem Cell, vol. 22; 2018. p. 252–261.e4.
ClinicalTrials.gov. 2018 [cited 2018 7th March]; Available from: https://clinicaltrials.gov/ct2/results?cond=CRISPR&Search=Apply&recrs=b&recrs=a&age_v=&gndr=&type=&rslt=.
Funding
JKYC is funded by Ministry of Heath’s National Medical Research Council (NMRC), Singapore (NMRC/CSA-SI/0008/2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yiping Fan and Jerry Kok Yen Chan declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genome Editing
Rights and permissions
About this article
Cite this article
Fan, Y., Chan, J.K.Y. Editing the Genome Ex Vivo Stem Cell Therapy. Curr Stem Cell Rep 4, 338–345 (2018). https://doi.org/10.1007/s40778-018-0148-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-018-0148-2