Abstract
Purpose of Review
Prematurity is a global health problem representing one of the most significant causes of morbidity and mortality in children. The concept of an artificial womb has been explored as an innovation which could substantially improve clinical outcomes in preterm patients by offering the ability to support growth and development of the neonate in a manner consistent with fetal physiology. This review will address the contemporary literature exploring the development of the artificial womb, with a focus on promising new breakthroughs in the field.
Recent Findings
Our laboratory has reported the development of an artificial womb incorporating the critical components of intrauterine physiology, namely sterile fluidic incubation of the isolated fetus as well as perfusion and gas exchange using a pumpless extracorporeal circuit. We have demonstrated support of fetal lambs for up to 4 weeks with stable hemodynamics and normal growth and development.
Summary
The artificial womb represents unprecedented potential for improvement in clinical outcomes in critically preterm infants. Recent studies have demonstrated promising results in preclinical animal models, supporting the translation of this technology to human clinical trials.
Similar content being viewed by others
Introduction
Despite advances in neonatal intensive care, prematurity remains an unsolved clinical challenge and represents a leading cause of infant morbidity and mortality [1]. Approximately 6% of all live births in the USA are considered extremely preterm (delivery at less than 28 weeks gestation), and the incidence of prematurity has slowly risen over the past decade [2]. Prematurity is thought to account for one third of infant mortality [3], and 80% of survivors born at 22 to 28 weeks gestation will suffer at least one major comorbidity [4•]. Neonatal care has evolved with the incorporation of protective ventilation strategies to minimize lung damage from gas-based respirators as well as the administration of surfactant and antenatal steroids, resulting in improvements in overall preterm outcomes. However, infants born at the cusp of viability at 22 to 25 weeks’ gestation continue to suffer devastating complications and very high rates of mortality. This is due in part to the fixed defect in surface area available for gas exchange in the lung which has not yet progressed from the canalicular to saccular stage of development [5]. The development of an extrauterine system supporting normal fetal growth and organ maturation for even a few weeks would be expected to significantly reduce both mortality as well as the burden of long-term comorbidities in surviving patients. The fundamental components of the artificial womb include sterile fluidic incubation, analogous to the intrauterine amniotic environment, as well as blood circulation and gas exchange driven by the innate fetal circulation, analogous to the umbilical-placental system. A number of preclinical animal models of the artificial womb have been reported in the literature with circuit design extrapolated from conventional extracorporeal membrane oxygenation (ECMO) technology, using pump-driven circuits to achieve fetal perfusion. These studies have reported varying degrees of success in maintaining the isolated fetus for brief experimental periods but have failed to achieve significant long-term support with meaningful growth and development.
Early Animal Models
The concept of extracorporeal oxygenation of the fetus is appealing due to the similarities to innate fetal physiology, in which gas exchange is performed by the umbilical-placental circulation. The first experimental animal models exploring extracorporeal oxygenation of the fetus were reported in the 1960s, with a series of short experiments in which fetal lambs were cannulated via the umbilical vessels and perfused by first-generation bubble membrane oxygenators with a total duration of support from 40 min to 2 days [6, 7]. These pioneering experiments were ultimately limited by overwhelming sepsis, circuit- and oxygenator-related complications, and cardiac failure. Thanks in part to substantial improvements in oxygenator technology, including the development of low-resistance hollow-fiber membrane technology, the duration of extrauterine fetal life support was extended to several weeks in studies employing conventional pump-driven ECMO circuits [8,9,10,11,12,13]. However, these studies were ultimately still limited by circulatory overload and cardiac failure, suggesting an unacceptable cardiac afterload imposed by these circuits, resulting in death of experimental animals and limiting duration of fetal support. The ideal interface for gas exchange in the artificial womb will permit the fetus to maintain circulation analogous to that achieved in the intact fetal umbilical-placental unit, with oxygenator perfusion determined by fetal cardiac output, leading to studies focusing on novel circuit design with elimination of pump-driven blood flow.
A simplified low-volume pumpless arteriovenous circuit offers several advantages over conventional ECMO technology including reduced priming and distribution volumes, shorter exposure of blood to thrombogenic surfaces, and the potential to achieve more physiologic regulation of blood flow and systemic pressures by the fetal heart itself. While a pumpless system has been accepted as the ideal paradigm to achieve the preservation of fetal physiology, earlier attempts to design a pumpless system for fetal or neonatal perfusion yielded discouraging results [14,15,16,17]. Awad et al. [14] described the use of a pumpless circuit in a series of lambs with surgically created congenital diaphragmatic hernias, with perfusion for up to 6 h but inadequate circuit flow rates and oxygenation levels to sustain ongoing support. Reoma et al. [15] reported their experience with a pumpless extracorporeal circuit using a hollow-fiber oxygenator and umbilical cannulation in four near-term lambs (GA 140), with animals supported for up to 4 hours before declining circuit flows and oxygenation resulted in early demise [15]. Mirua et al. [16] undertook a study of five animals supported on a circuit with similar design to that described by Reoma et al., and observed a progressive lactic acidosis resulting in cardiac failure and death after an average of 18 h of support. Schoberer et al. reported the development of a miniaturized low-volume oxygenator studied over a 6-h period of support [17]; however, all animals developed progressive hypotension and metabolic acidosis, with three of the seven experimental animals ultimately requiring catecholamines over the course of the runs.
These discouraging preliminary results led to the design and implementation of experimental models including avoidance of umbilical arterial cannulation by the use of a conventional pump-supported veno-venous ECMO circuit, and replacement of a fluid environment by a fluid-filled endotracheal tube or mechanical ventilation [18, 19••]. While these modifications have supported survival for as long as 1 week on extracorporeal support, they do not replicate normal fetal lung physiology or maintain a normal fetal circulation. Thus, they have moved further from normal fetal physiology rather than pursuing the ideal goal of replicating it.
The Artificial Womb: Long-Term Pumpless Fetal Support in a Fluid Environment
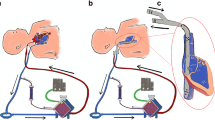
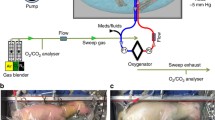
Based on the principles of maintaining normal fetal circulation and the advances in low-resistance hollow-fiber oxygenator technology, we conceptualized the design of an artificial womb incorporating a simplified low-resistance arteriovenous pumpless circuit with minimal priming volumes and surface area, with flow driven by the native cardiac output and endogenous arteriovenous pressure gradient. Circuit design included an oxygenator with near-zero measured resistance and a priming volume of less than 80 mL (Maquet Quadrox-ID Pediatric Oxygenator: Maquet Cardiopulmonary AG, Rastatt, Germany), with short segments of tubing to deliver blood from the arterial circulation to the oxygenator with return to the venous circulation, and with incorporation of anti-thrombogenic surfacing for all cannulas, tubing, and internal oxygenator surfacing [20•].
While we recognized that the ideal vascular interface for the oxygenator circuit would be the umbilical cord, initial experiments employed a carotid artery/jugular vein cannulation strategy in near-term lambs (120 to 140 days, term 145 days) to avoid umbilical spasm. After accessing the fetus by creation of a hysterotomy, cannulation of the target vessels was performed, with establishment of circuit flow to the extracorporeal oxygenator prior to transfer of the fetus into the fluid environment. From our earliest pilot experiments, animals supported on the artificial womb have demonstrated remarkable hemodynamic stability with no evidence of acidosis or heart failure. Sepsis secondary to contamination of the incubator fluid was observed at high rates in our early studies, precluding runs in excess of more than 1 to 2 weeks. Our early models for fluidic incubation relied on recirculation and filtration of synthetic amniotic fluid, which resulted in high rates of bacterial growth and early onset of bacteremia and sepsis. Through various modifications, this evolved to a closed fluid circuit with the lamb encased in a “Biobag” with continuous exchange of the synthetic amniotic fluid. This allowed continuous turnover of the fluid multiple times within a 24-h period, analogous to the exchange of the amniotic fluid in utero [21].
Having demonstrated stable long-term support of near-term fetal lambs in the artificial womb, we undertook study of animals at a stage of prematurity analogous to the clinical target population. Late canalicular lung development is observed at 22–24 weeks gestation in the human infant, corresponding to lambs at 100–115 days gestation. Initial experiences in 110-day gestational age lambs supported on the circuit demonstrated diminishing circuit flow and systemic blood pressure as well as progressive edema, suggesting the near-systolic pressures of venous return via the superior vena cava in this circuit imposed an unacceptable preload on the fetal heart. Normalization of the arteriovenous pressure gradient was achieved by cannulation of the umbilical vein beyond the umbilical fascia and maintenance of carotid artery inflow. With these modifications, i.e., the closed fluid exchange system and umbilical vein cannulation, we were able to maintain physiologic stability for 20–26 days in five lambs that were developmentally equivalent to 22–23-week gestation human fetuses (108–113 days gestation).
However, despite the physiologic and metabolic stability of these lambs, it was not a fully physiologic system. The critical deficit limiting the achievement of truly normal physiology was subphysiologic circuit flow. The normal placenta receives 150–200 mL/kg/min of blood flow whereas, because of the inherent limitation of carotid artery blood flow, we were achieving only 70–100 mL/kg/min. This resulted in the necessity for a number of non-physiologic compensations to match oxygen delivery with oxygen consumption. These included transfusion with adult blood to a higher hemoglobin level, the maintenance of higher post membrane PaO2, and the use of sedation to reduce fetal oxygen consumption. In addition, carotid artery cannulation had numerous disadvantages, including the need for an operation on the fetus to place and remove the cannula, the secondary ligation of the common carotid with its potential impact on cerebral blood flow and brain development, and the potential for a decannulation event necessitating prophylactic sedation. The concern with the use of the umbilical vessels was umbilical vascular spasm and erosion or aneurysmal degeneration of the vessels. While other investigators have utilized the umbilical vessels, all have advanced cannulas through the umbilical vessels into the central circulation. This avoids spasm but increases resistance through the circuit which also prevents physiologic circuit flow. In addition, because of the tortuosity of the human umbilical arteries, cannulation to the aorta is not possible for large cannulas without significant risk. We therefore developed a completely new technique for umbilical cannulation which maintains a length of native umbilical cord between the cannula tips and the abdominal wall and eliminates any significant length of cannula within the cord vessels. This functional end adapter system minimized the possibility of cord occlusion from kinking or angulation, prevented any deterioration of the cord vessels, and most importantly, allowed truly physiologic “placental” blood flow. It also eliminated the concern for decannulation events allowing the avoidance of any sedation. Umbilical cannulation resulted in the provision of stable support of extremely preterm lambs (105 to 110 days gestation) for up to 4 weeks with circuit flow rates equivalent to normal physiologic flow to the placenta and growth, oxygen delivery, and organ maturation within expected norms. Daily echocardiography confirmed maintenance of the fetal cardiac circulation with good cardiac contractility and function. Significant growth and development was qualitatively observed over the course of the runs, with animals opening their eyes, becoming more active, displaying normal breathing and swallowing movements, and growth of wool over the course of incubation. Tissue histology after 4 weeks of support demonstrated evidence of normal growth and development, with lung morphometric analysis showing progression from the canalicular to saccular stage of development in parallel with age-matched normal control specimens, and brains showing normal histology, myelination, and weight, with no evidence of hemorrhage or infarct on whole sectioning or MRI. Animals have normal ventilator function compared to age-matched controls delivered by cesarean section. Finally, animals can be successfully transitioned off of the device with a very short period of ventilation, with apparent normal outcomes up to 6 months of age.
Conclusions
These results demonstrate that extreme premature fetal lambs can be supported in the artificial womb for up to 4 weeks without apparent physiologic derangement or organ failure. In addition, we have demonstrated transition to postnatal life after prolonged support in the artificial womb. There are a number of key features of the current artificial womb system that contribute to this success. The first is an extremely low-resistance oxygenator incorporated in a pumpless circuit with low surface area and priming volumes, connected to the fetal vasculature in an arterial to venous orientation, resulting in highly efficient gas exchange. Additionally, the fetus in the artificial womb demonstrates spontaneous fluid breathing and swallowing movements analogous to normally observed fetal activity in utero. This has resulted in normal lung development and maturation by histologic and functional criteria. The ability to maintain a sterile amniotic fluid environment over 4 weeks of support was a critical advance in achieving normal lung development supporting normal fetal breathing and intrabronchial pressures.
Importantly, the recent report of the EVE (Ex-Vivo uterine Environment) model of an artificial womb provides independent validation of a number of the observations made by our group. In this system, trans-umbilical cannulation into the central circulation is performed in fetal lambs with attachment to two hollow membrane oxygenators arranged in parallel orientation and maintained in a sterile fluidic bath of synthetic amniotic fluid [22]. In the most recent report from this group, although physiologic circuit flow was not achieved, five of the six experimental animals were successfully maintained for 1 week with stable hemodynamics and no incidence of infection [23]. While longer experiments of up to 4 weeks will be required to make direct comparisons to the outcomes observed in our laboratory, these promising preliminary results support the ongoing efforts to further refine and develop the artificial womb model using exclusively umbilical cannulation and sterile fluidic incubation as the most physiologic approximation of intrauterine conditions.
The implications of the artificial womb extend beyond the support of extremely premature infants. Potential clinical applications may include treatment of fetal growth retardation related to placental insufficiency and salvage of preterm infants threatening to deliver after fetal intervention or due to chorioamnionitis. An additional application would be the possibility for early delivery of infants affected by congenital malformations of the heart, lung, and diaphragm for early correction or therapy prior to the institution of gas ventilation. Fetal stem cell or gene therapy could be facilitated by removing the possibility for maternal exposure and enabling direct delivery of therapeutic agents to the isolated fetus. Finally, the artificial womb offers an experimental model for addressing fundamental questions related to the role of the placenta in fetal development. Long-term physiologic maintenance of a fetus separated from the maternal-placental unit has now been achieved, making it possible to study the relative contribution of the placenta itself to fetal maturation [20•]. The artificial womb therefore represents a powerful new tool for numerous research and clinical applications.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
March of Dimes; Partnership for Maternal, Newborn, and Child Health Save the Children, WHO. Born too soon: the global action report on preterm birth. Geneva: World Health Organization; 2012.
Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg. 2015;120(6):1337–51. https://doi.org/10.1213/ANE.0000000000000705.
Callaghan WM, MF MD, Rasmussen SA, et al. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–73. https://doi.org/10.1542/peds.2006-0860.
• Anderson JG, Baer RJ, Partridge JC, et al. Survival and major morbidity of extremely preterm infants: a population-based study. Pediatrics. 2016;138(1):e20154434. This study illustrates the unmet need of extreme prematurity with recent assessment of associated mortality and morbidity
Coalson JJ. Pathology of new brochopulmonary dysplasia. Semin Neonatol. 2003;8(1):73–81. https://doi.org/10.1016/S1084-2756(02)00193-8.
Maynes EA, Callaghan JC. A new method of oxygenation: a study of its use in respiratory support and the artificial placenta. Ann Surg. 1963;158(4):537–42. https://doi.org/10.1097/00000658-196310000-00003.
Zapol WM, Kolobow T, Pierce JG, et al. Artificial placenta: two days of total extrauterine support of the isolated premature lamb. Science. 1969;166(3905):617–8. https://doi.org/10.1126/science.166.3905.617.
Kuwabara Y, Okai T, Imanishi Y, et al. Development of extrauterine fetal incubation system using extracorporeal membrane oxygenator. Artif Organs. 1986;11:224–77.
Kuwabara Y, Okai T, Kozuma S, et al. Artificial placenta: long-term extrauterine incubation of isolated goat fetuses. Artif Organs. 1989;13:527–31.
Unno N, Kuwabara Y, Okai T, et al. Development of an artificial placenta: survival of isolated goat fetuses for up to three weeks with umbilical arteriovenous extracorporeal oxygenation. Artif Organs. 1993;17:996–1003.
Unno N, Baba K, Kozuma S, et al. An evaluation of the system to control blood flow in maintaining goat fetuses on arterio-venous extracorporeal membrane oxygenation: a novel approach to the development of an artificial placenta. Artif Organs. 1997;21:1239–46.
Yasufuku M, Hisano K, Sakata M, et al. Arterio-venous extracorporeal membrane oxygenation of fetal goat incubated in artificial amniotic fluid (artificial placenta): influence on lung growth and maturation. J Pediatr Surg. 1998;33:442–8.
Pak SC, Song CH, So GY, Jang CH, Lee KH, Kim JY. Extrauterine incubation of fetal goats applying the extracorporeal membrane oxygenation via umbilical artery and vein. J Korean Med Sci. 2002;17(5):663–8. https://doi.org/10.3346/jkms.2002.17.5.663.
Awad JA, Cloutier R, Fournier L, et al. Pumpless respiratory assistance using a membrane oxygenator as an artificial placenta: a preliminary study in newborn and preterm lambs. J Investig Surg. 1995;8:21–30.
Reoma JL, Rojas A, Kim AC, et al. Development of an artificial placenta I: pumpless arterio-venous extracorporeal life support in a neonatal sheep model. J Pediatr Surg. 2009;44:53–9.
Mirua Y, Matsuda T, Funakubo A, et al. Novel modification of an artificial placenta: pumpless arteriovenous extracorporeal life support in a premature lamb model. Pediatr Res. 2002;72:490–4.
Schoberer M, Arens J, Erben A, et al. Miniaturization: the clue to clinical application of the artificial placenta. Artif Organs. 2014;38:208–14.
Gray BW, El-Sabbagh A, Zakem SJ, et al. Development of an artificial placenta V: 70h veno-venous extracorporeal life support after ventilatory failure in premature lambs. J Pediatr Surg. 2013;48(1):145–53.
• Bryner B, Gray B, Perkins E, et al. An extracorporeal placenta supports extremely premature lambs for one week. J Pediatr Surg. 2015;50(1):44–9. This study describes an alternate approach for non-physiologic support of the extreme premature infant utilizing veno-venous ECMO and tracheal occlusion
•• Partridge EA, Davey MG, Hornick MA, et al. An extra-uterine system to physiologically support the extreme preterm lamb. Nat Comm. 2017;8:15112. https://doi.org/10.1038/ncomms15112. This study describes the first physiologic system capable of long-term support of the extreme premature lamb.
Beall MH, van den Wijngaard JPHM, van Gemert MJC, Ross MG. Amniotic fluid water dynamics. Placenta. 2007;28(8–9):816–23. https://doi.org/10.1016/j.placenta.2006.11.009.
Miura Y, Saito M, Usuda H, et al. Ex-vivo uterine environment (EVE) therapy induced limited fetal inflammation in a premature lamb model. PLoS One. 2015;10(10):1–17.
Usuda H, Watanabe S, Miura Y, et al. Successful maintenance of key physiological parameters in preterm lambs treated with ex vivo uterine environment therapy for a period of 1 week. Am J Obstet Gynecol. 2017;217:457 e1–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Emily A. Partridge reports she has a patent WO2014145494 A1 issued to Children’s Hospital of Philadelphia.
Marcus G. Davey reports he has a patent WO2014145494 A1 issued and a patent PCT/US16/38045 pending.
Alan W. Flake reports he has a patent WO2014145494 A1 issued and a patent PCT/US16/38045 pending.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Prenatal Therapies
Rights and permissions
About this article
Cite this article
Partridge, E.A., Davey, M.G. & Flake, A.W. Development of the Artificial Womb. Curr Stem Cell Rep 4, 69–73 (2018). https://doi.org/10.1007/s40778-018-0120-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-018-0120-1