Abstract
Purpose of Review
Celiac disease is a common chronic autoimmune condition for which the only therapy currently available is strict adherence to a gluten-free diet for life. Although the diet is effective in reversing the intestinal mucosal changes, it is cumbersome to follow, is associated with some dietary deficiencies, is less palatable, and has significant quality of life implications. For all these reasons, alternatives to the gluten-free diet would greatly benefit people with celiac disease.
Recent Findings
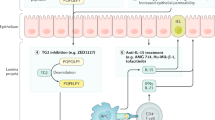
A better understanding of the pathophysiology of celiac disease has led to possible new treatments that target various steps in the development of the disease. These include intraluminal digestive enzymes and peptide-binding agents that render gluten non-toxic, drugs that modulate tight junctions between enterocytes or interfere with the inflammatory cascade that causes mucosal destruction, and agents designed to induce immune tolerance to gluten.
Summary
Although several of these new therapeutic agents currently under investigation are showing some promise, they still need to demonstrate they are as effective and safe as the gluten-free diet before they can be recommended as an acceptable alternative for treatment of people with celiac disease. The gluten-free diet remains the only proven safe and effective treatment for celiac disease.
Similar content being viewed by others
Abbreviations
- CD:
-
Celiac disease
- GFD:
-
Gluten-free diet
- tTG:
-
Tissue transglutaminase
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62(1):43–52. https://doi.org/10.1136/gutjnl-2011-301346.
• Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(6):823–36 e2. https://doi.org/10.1016/j.cgh.2017.06.037 This meta-analysis demonstrates that while prevalence of celiac disease may vary by country, it remains high worldwide and is increasing over time.
Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42(8):587–95. https://doi.org/10.3109/07853890.2010.505931.
Yuan J, Zhou C, Gao J, Li J, Yu F, Lu J, et al. Prevalence of celiac disease autoimmunity among adolescents and young adults in China. Clin Gastroenterol Hepatol. 2017;15(10):1572–9 e1. https://doi.org/10.1016/j.cgh.2017.04.025.
Catassi C, Gatti S, Lionetti E. World perspective and celiac disease epidemiology. Dig Dis. 2015;33(2):141–6. https://doi.org/10.1159/000369518.
Kupfer SS, Jabri B. Pathophysiology of celiac disease. Gastrointest Endosc Clin N Am. 2012;22(4):639–60. https://doi.org/10.1016/j.giec.2012.07.003.
•• Hill I, Fasano A, Guandalini S, Hoffenberg E, Levy J, Reilly N, et al. NASPGHAN Clinical report on the diagnosis and treatment of gluten-related disorders. Journal of Pediatric Gastroenterology and Nutrition. 2016;63(1):156–65. https://doi.org/10.1097/MPG.0000000000001216 These are the North American guidelines for the diagnosis of celiac disease which include recommendations for biopsy to establish the diagnosis.
•• Husby S, Koletzko S, Korponay-Szabo I, Kurppa K, Mearin ML, Ribes-Koninckx C, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70(1):141–56. https://doi.org/10.1097/MPG.0000000000002497 These are the European guidelines for the diagnosis of celiac disease which include recommendations and guidance for a biopsy-free diagnosis.
Thomas B, Bishop J. British Dietetic Association. Manual of dietetic practice. 4th ed. Oxford. Blackwell Pub.: Ames; 2007.
Studerus D, Hampe EI, Fahrer D, Wilhelmi M, Vavricka SR. Cross-Contamination with gluten by using kitchen utensils: fact or fiction? J Food Prot. 2018;81(10):1679–84. https://doi.org/10.4315/0362-028X.JFP-17-383.
• Weisbrod VM, Silvester JA, Raber C, McMahon J, Coburn SS, Kerzner B. Preparation of gluten-free foods alongside gluten-containing food may not always be as risky for celiac patients as diet guides suggest. Gastroenterology. 2020;158(1):273–5. https://doi.org/10.1053/j.gastro.2019.09.007 This study is able to provide evidence that food preparation recommendations for a gluten-free diet may not need to be as strict as previously thought.
Miller K, McGough N, Urwin H. Catering gluten-free when simultaneously using wheat flour. J Food Prot. 2016;79(2):282–7. https://doi.org/10.4315/0362-028X.JFP-15-213.
Food Labeling. Gluten-free labeling of foods. Dept of Health and Human Services, FDA Final Rule. Docket No. FDA-2005-N-0404.2013.
Food Labeling. Gluten-free labeling of fermented or hydrolyzed foods. Dept of Health and Human Services, FDA Final Rule, Docket No. FDA-2014-N-1021. 2020.
Kulai T, Rashid M. Assessment of nutritional adequacy of packaged gluten-free food products. Can J Diet Pract Res. 2014;75(4):186–90. https://doi.org/10.3148/cjdpr-2014-022.
Zuccotti G, Fabiano V, Dilillo D, Picca M, Cravidi C, Brambilla P. Intakes of nutrients in Italian children with celiac disease and the role of commercially available gluten-free products. J Hum Nutr Diet. 2013;26(5):436–44. https://doi.org/10.1111/jhn.12026.
Amirikian K, Sansotta N, Guandalini S, Jericho H. Effects of the gluten-free diet on body mass indexes in pediatric celiac patients. J Pediatr Gastroenterol Nutr. 2019;68(3):360–3. https://doi.org/10.1097/MPG.0000000000002190.
Olsson C, Lyon P, Hornell A, Ivarsson A, Sydner YM. Food that makes you different: the stigma experienced by adolescents with celiac disease. Qual Health Res. 2009;19(7):976–84. https://doi.org/10.1177/1049732309338722.
Rashid M, Cranney A, Zarkadas M, Graham ID, Switzer C, Case S, et al. Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics. 2005;116(6):e754–9. https://doi.org/10.1542/peds.2005-0904.
MacCulloch K, Rashid M. Factors affecting adherence to a gluten-free diet in children with celiac disease. Paediatr Child Health. 2014;19(6):305–9. https://doi.org/10.1093/pch/19.6.305.
Mager DR, Marcon M, Brill H, Liu A, Radmanovich K, Mileski H, et al. Adherence to the gluten-free diet and health-related quality of life in an ethnically diverse pediatric population with celiac disease. J Pediatr Gastroenterol Nutr. 2018;66(6):941–8. https://doi.org/10.1097/MPG.0000000000001873.
Shah S, Akbari M, Vanga R, Kelly CP, Hansen J, Theethira T, et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am J Gastroenterol. 2014;109(9):1304–11. https://doi.org/10.1038/ajg.2014.29.
Yoosuf S, Makharia GK. Evolving therapy for celiac disease. Front Pediatr. 2019;7:193. https://doi.org/10.3389/fped.2019.00193.
Murray JA, Kelly CP, Green PHR, Marcantonio A, Wu TT, Maki M, et al. No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology. 2017;152(4):787–98 e2. https://doi.org/10.1053/j.gastro.2016.11.004.
Syage JA, Murray JA, Green PHR, Khosla C. Latiglutenase improves symptoms in seropositive celiac disease patients while on a gluten-free diet. Dig Dis Sci. 2017;62(9):2428–32. https://doi.org/10.1007/s10620-017-4687-7.
Syage JA, Green PHR, Khosla C, Adelman DC, Sealey-Voyksner JA, Murray JA. Latiglutenase treatment for celiac disease: symptom and quality of life improvement for seropositive patients on a gluten-free diet. GastroHep. 2019;1(6):293–301. https://doi.org/10.1002/ygh2.371.
Wolf C, Siegel JB, Tinberg C, Camarca A, Gianfrani C, Paski S, et al. Engineering of Kuma030: a gliadin peptidase that rapidly degrades immunogenic gliadin peptides in gastric conditions. J Am Chem Soc. 2015;137(40):13106–13. https://doi.org/10.1021/jacs.5b08325.
Salden BN, Monserrat V, Troost FJ, Bruins MJ, Edens L, Bartholome R, et al. Randomised clinical study: aspergillus niger-derived enzyme digests gluten in the stomach of healthy volunteers. Aliment Pharmacol Ther. 2015;42(3):273–85. https://doi.org/10.1111/apt.13266.
Mohan Kumar BV, Vijaykrishnaraj M, Kurrey NK, Shinde VS, Prabhasankar P. Prolyl endopeptidase-degraded low immunoreactive wheat flour attenuates immune responses in Caco-2 intestinal cells and gluten-sensitized BALB/c mice. Food Chem Toxicol. 2019;129:466–75. https://doi.org/10.1016/j.fct.2019.05.011.
Konig J, Holster S, Bruins MJ, Brummer RJ. Randomized clinical trial: Effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci Rep. 2017;7(1):13100. https://doi.org/10.1038/s41598-017-13587-7.
Tack GJ, van de Water JM, Bruins MJ, Kooy-Winkelaar EM, van Bergen J, Bonnet P, et al. Consumption of gluten with gluten-degrading enzyme by celiac patients: a pilot-study. World J Gastroenterol. 2013;19(35):5837–47. https://doi.org/10.3748/wjg.v19.i35.5837.
Valitutti F, Fasano A. Breaking down barriers: how understanding celiac disease pathogenesis informed the development of novel treatments. Dig Dis Sci. 2019;64(7):1748–58. https://doi.org/10.1007/s10620-019-05646-y.
Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26(5):757–66. https://doi.org/10.1111/j.1365-2036.2007.03413.x.
Leffler DA, Kelly CP, Abdallah HZ, Colatrella AM, Harris LA, Leon F, et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol. 2012;107(10):1554–62. https://doi.org/10.1038/ajg.2012.211.
Kelly CP, Green PH, Murray JA, Dimarino A, Colatrella A, Leffler DA, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37(2):252–62. https://doi.org/10.1111/apt.12147.
Leffler DA, Kelly CP, Green PH, Fedorak RN, DiMarino A, Perrow W, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology. 2015;148(7):1311–9 e6. https://doi.org/10.1053/j.gastro.2015.02.008.
Alhassan E, Yadav A, Kelly CP, Mukherjee R. Novel nondietary therapies for celiac disease. Cell Mol Gastroenterol Hepatol. 2019;8(3):335–45. https://doi.org/10.1016/j.jcmgh.2019.04.017.
Pinier M, Verdu EF, Nasser-Eddine M, David CS, Vezina A, Rivard N, et al. Polymeric binders suppress gliadin-induced toxicity in the intestinal epithelium. Gastroenterology. 2009;136(1):288–98. https://doi.org/10.1053/j.gastro.2008.09.016.
McCarville JL, Nisemblat Y, Galipeau HJ, Jury J, Tabakman R, Cohen A, et al. BL-7010 demonstrates specific binding to gliadin and reduces gluten-associated pathology in a chronic mouse model of gliadin sensitivity. PLoS One. 2014;9(11):e109972. https://doi.org/10.1371/journal.pone.0109972.
Xia J, Bergseng E, Fleckenstein B, Siegel M, Kim CY, Khosla C, et al. Cyclic and dimeric gluten peptide analogues inhibiting DQ2-mediated antigen presentation in celiac disease. Bioorg Med Chem. 2007;15(20):6565–73. https://doi.org/10.1016/j.bmc.2007.07.001.
Kapoerchan VV, Wiesner M, Overhand M, van der Marel GA, Koning F, Overkleeft HS. Design of azidoproline containing gluten peptides to suppress CD4+ T-cell responses associated with celiac disease. Bioorg Med Chem. 2008;16(4):2053–62. https://doi.org/10.1016/j.bmc.2007.10.091.
Kapoerchan VV, Wiesner M, Hillaert U, Drijfhout JW, Overhand M, Alard P, et al. Design, synthesis and evaluation of high-affinity binders for the celiac disease associated HLA-DQ2 molecule. Mol Immunol. 2010;47(5):1091–7. https://doi.org/10.1016/j.molimm.2009.10.036.
Tye-Din JA, Stewart JA, Dromey JA, Beissbarth T, van Heel DA, Tatham A, et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med. 2010;2(41):41ra51. https://doi.org/10.1126/scitranslmed.3001012.
Goel G, King T, Daveson AJ, Andrews JM, Krishnarajah J, Krause R, et al. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: two randomised, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol Hepatol. 2017;2(7):479–93. https://doi.org/10.1016/S2468-1253(17)30110-3.
Daveson AJM, Ee HC, Andrews JM, King T, Goldstein KE, Dzuris JL, et al. Epitope-specific immunotherapy targeting CD4-positive T cells in celiac disease: safety, pharmacokinetics, and effects on intestinal histology and plasma cytokines with escalating dose regimens of Nexvax2 in a randomized, double-blind, placebo-controlled phase 1 study. EBioMedicine. 2017;26:78–90. https://doi.org/10.1016/j.ebiom.2017.11.018.
Truitt KE, Daveson AJM, Ee HC, Goel G, MacDougall J, Neff K, et al. Randomised clinical trial: a placebo-controlled study of subcutaneous or intradermal NEXVAX2, an investigational immunomodulatory peptide therapy for coeliac disease. Aliment Pharmacol Ther. 2019;50(5):547–55. https://doi.org/10.1111/apt.15435.
Pearson RM, Podojil JR, Shea LD, King NJC, Miller SD, Getts DR. Overcoming challenges in treating autoimmuntity: development of tolerogenic immune-modifying nanoparticles. Nanomedicine. 2019;18:282–91. https://doi.org/10.1016/j.nano.2018.10.001.
Freitag TL, Podojil JR, Pearson RM, Fokta FJ, Sahl C, Messing M, et al. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of celiac disease. Gastroenterology. 2020;158(6):1667–81 e12. https://doi.org/10.1053/j.gastro.2020.01.045.
Kelly CP, Murray J, Leffler D, Bledsoe A, Smithson G, Podojil JR et al., editors. CNP-101 prevents gluten challenge induced immune activation in adults with celiac disease. United European Gastroenterology Week; 2019 10/22/2019; Barcelona, Spain.
Lahdeaho ML, Scheinin M, Vuotikka P, Taavela J, Popp A, Laukkarinen J, et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol Hepatol. 2019;4(12):948–59. https://doi.org/10.1016/S2468-1253(19)30264-X.
Cellier C, Bouma G, van Gils T, Khater S, Malamut G, Crespo L, et al. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: a phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol Hepatol. 2019;4(12):960–70. https://doi.org/10.1016/S2468-1253(19)30265-1.
Yokoyama S, Perera PY, Waldmann TA, Hiroi T, Perera LP. Tofacitinib, a janus kinase inhibitor demonstrates efficacy in an IL-15 transgenic mouse model that recapitulates pathologic manifestations of celiac disease. J Clin Immunol. 2013;33(3):586–94. https://doi.org/10.1007/s10875-012-9849-y.
Theron M, Bentley D, Nagel S, Manchester M, Gerg M, Schindler T, et al. Pharmacodynamic monitoring of RO5459072, a small molecule inhibitor of cathepsin S. Front Immunol. 2017;8:806. https://doi.org/10.3389/fimmu.2017.00806.
Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6(5):394–403. https://doi.org/10.1038/nri1838.
Mukewar SS, Sharma A, Rubio-Tapia A, Wu TT, Jabri B, Murray JA. Open-capsule budesonide for refractory celiac disease. Am J Gastroenterol. 2017;112(6):959–67. https://doi.org/10.1038/ajg.2017.71.
Therrien A, Silvester JA, Leonard MM, Leffler DA, Fasano A, Kelly CP. Enteric-release budesonide may be useful in the management of non-responsive celiac disease. Dig Dis Sci. 2020. https://doi.org/10.1007/s10620-020-06454-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ivor Hill, MD, receives royalties from UpToDate as a chapter author. Brandon Sparks declares that he has no conflict of interest. Tracy Ediger declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Gastroenterology
Rights and permissions
About this article
Cite this article
Sparks, B., Hill, I. & Ediger, T. Going Beyond Gluten-Free: a Review of Potential Future Therapies for Celiac Disease. Curr Treat Options Peds 7, 17–31 (2021). https://doi.org/10.1007/s40746-020-00217-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40746-020-00217-0