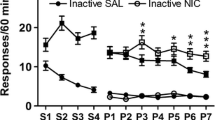
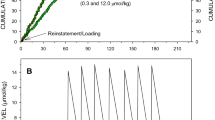
Abstract
In 2 experiments, we investigated the utility of a progressive-duration schedule as an assay for measuring drug effects. In both experiments, rats responded on a schedule of reinforcement in which food delivery was contingent upon response duration. Response requirements increased after each reinforcer delivery in a fashion similar to that of progressive-ratio schedules. Naloxone (1.0, 3.0, and 10.0 mg/kg) produced dose-dependent decreases in breaking points, a finding consistent with those of previous studies demonstrating that opioid antagonists may reduce the reinforcing efficacy of highly palatable foods in sated organisms. In a second experiment, caffeine (3.0, 6.25, and 12.5 mg/kg) sometimes produced increases in breaking points but reduced efficiency by increasing the proportions of lever presses that were too short to satisfy reinforcer requirements. Food deprivation had similar effects. The progressive-duration schedule shows promise as an assay sensitive to motivational aspects of behavior; however, distinguishing the rate-altering effects of drugs from their effects on motivating operations may pose interpretation challenges.






Similar content being viewed by others
Notes
We prefer the term progressive duration schedule, as naming the schedule according to topography would require separate names for each response form studied (e.g., progressive nose poke and progressive plunger pull schedules).
References
Bailey, M. R., Jensen, G., Taylor, K., Mezias, C., Williamson, C., Silver, R., . . . Balsam, P. D. (2015). A novel strategy for dissecting goal-directed action and arousal components of motivated behavior with a progressive hold-down task. Behavioural Neuroscience, 129, 269–280. doi: https://doi.org/10.1037/bne0000060.
Bailey, M. R., Williamson, C., Mezias, C., Winiger, V., Silver, R., Balsam, P. D., & Simpson, E. H. (2016). The effects of pharmacological modulation of the serotonin 2C receptor on goal-directed behavior in mice. Psychopharmacology, 233, 615–624. https://doi.org/10.1007/s00213-015-4135-3.
Barbano, M. F., & Cador, M. (2006). Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opiodergic drugs. Neuropsychopharmacology, 31, 1371–1381. https://doi.org/10.1038/sj.npp.1300908.
Barbano, M. F., & Cador, M. (2007). Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology, 191, 497–506. https://doi.org/10.1007/s00213-006-0521-1.
Barbano, M. F., Le Saux, M., & Cador, M. (2009). Involvement of dopamine and opioids in the motivation to eat: Influence of palatability, homeostatic state, and behavioral paradigms. Psychopharmacology, 203, 475–487. https://doi.org/10.1007/s00213-008-1390-6.
Clearly, J., Weldon, D. T., O’Hare, E., Billington, C., & Levine, A. S. (1996). Naloxone effects on sucrose-motivated behavior. Psychopharmacology, 126, 110–114. https://doi.org/10.1007/bf02246345.
Covarrubias, P., & Aparicio, C. F. (2008). Effects of reinforcer quality and step size on rats’ performance under progressive ratio schedules. Behavioural Processes, 78, 246–252. https://doi.org/10.1016/j.beproc.2008.02.001.
Donny, E. C., Chaudhri, N., Cauggiula, A. R., Evans-Martin, F. F., Booth, S., Gharib, M. A., . . . Sved, A. F. (2003). Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology, 169, 68–76. doi: https://doi.org/10.1007/s00213-003-1473-3.
Faustman, W. O., & Fowler, S. C. (1981). Use of operant response duration to distinguish the effects of haloperidol from nonreward. Pharmacology Biochemistry and Behavior, 15, 327–329. https://doi.org/10.1016/0091-3057(81)90196-9.
Ferguson, S. A., & Paule, M. G. (1992). Acute effects of chlorpromazine in a monkey operant behavioral test battery. Pharmacology Biochemistry and Behavior, 42, 333–341. https://doi.org/10.1016/0091-3057(92)90536-O.
Fowler, S. C., Skjoldager, P. D., Ruey-Ming, L., Chase, M., & Johnson, J. S. (1991). Distinguishing between haloperidol’s and decamethonium’s disruptive effects on operant behavior in rats: Use of measurements that complement response rate. Journal of the Experimental Analysis of Behavior, 56, 239–260. https://doi.org/10.1901/jeab.1991.56-239.
Fredholm, B. B., Bättig, K., Holmén, J., Nehlig, A., & Zvartau, E. E. (1999). Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological Reviews, 51, 83–133.
Glass, M. J., O’Hare, E., Clearly, J. P., Billington, C. J., & Levine, A. S. (1999). The effect of naloxone on food-motivated behavior in the obese Zucker rat. Psychopharmacology, 141, 378–384. https://doi.org/10.1007/s002130050847.
Gulotta, K., & Byrne, T. (2015). A progressive-duration schedule of reinforcement. Behavioral Processes, 121, 93–97. https://doi.org/10.1016/j.beproc.2015.10.022.
Hayward, M., & Low, M. J. (2001). The effect of naloxone on operant behavior for food reinforcers in DBA/2 mice. Brain Research Bulletin, 56, 537–543. https://doi.org/10.1016/s0361-9230(01)00626-8.
Higgs, S., Barber, D. J., Cooper, A. J., & Terry, P. (2005). Differential effects of two cannabinoid receptor agonists on progressive ratio responding for food and free-feeding in rats. Behavioural Pharmacology, 16, 389–393. https://doi.org/10.1097/00008877-200509000-00011.
Hodos, W. (1961). Progressive ratio as a measure of reward strength. Science, 134, 943–944. https://doi.org/10.1126/science.134.3483.943.
Hudzik, T. J., & McMillan, D. E. (1994). Drug effects on response duration differentiation I: Differential effects of drugs of abuse. Psychopharmacology, 114, 620–627. https://doi.org/10.1007/bf02244993.
Jarmolowicz, D. P., & Lattal, K. A. (2010). On distinguishing progressively increasing response requirements for reinforcement. The Behavior Analyst, 33, 119–125.
Jones, C. A., LeSage, M., Sundby, S., & Poling, A. (1995). Effects of cocaine in pigeons responding under a progressive-ratio schedule of food delivery. Pharmacology Biochemistry and Behavior, 50, 527–531. https://doi.org/10.1016/0091-3057(94)00333-5.
Levine, A. S., & Billington, C. J. (2004). Opioids as agents of reward-related feeding: A consideration of the evidence. Physiology and Behavior, 82, 57–61. https://doi.org/10.1016/j.physbeh.2004.04.032.
Macenski, M. J., Schaal, D. W., Clearly, J., & Thompson, T. (1994). Changes in food-maintained progressive-ratio responding of rats following chronic buprenorphine or methadone administration. Pharmacology Biochemistry and Behavior, 47, 379–383. https://doi.org/10.1016/0091-3057(94)90027-2.
Marin, M. T., Zancheta, R., Paro, A. H., Posssie, A. P., Cruz, F. C., & Planeta, C. S. (2011). Comparison of caffeine-induced locomotor activity between adolescent and adult rats. European Journal of Pharmacology, 660, 363–367. https://doi.org/10.1016/j.ejphar.2011.03.052.
McClure, G. Y., Hardwick, W. C., & McMillan, D. E. (2000). Effect of drugs on response-duration differentiation VII: Response-force requirements. Journal of the Experimental Analysis of Behavior, 74, 295–309. https://doi.org/10.1901/jeab.2000.74-295.
Morgan, D., Liu, Y., Oleson, E. B., & Roberts, D. C. S. (2009). Cocaine self-administration on a hold-down schedule of reinforcement in rats. Psychopharmacology, 201, 601–609. https://doi.org/10.1007/s00213-008-1328-z.
Olszewski, P. K., & Levine, A. S. (2007). Central opioids and consumption of sweet tastants: When reward outweighs homeostasis. Physiology and Behavior, 91, 506–512. https://doi.org/10.1016/j.physbeh.2007.01.011.
Peck, S., & Byrne, T. (2016). Demand in rats responding under duration-based schedules of reinforcement. Behavioural Processes, 128, 47–52. https://doi.org/10.1016/j.beproc.2016.04.002.
Retzbach, E. P., Dholakia, P. H., & Duncan-Vaidya, E. A. (2014). The effect of daily caffeine exposure on lever-pressing for sucrose and c-Fos expression in the nucleus accumbens in the rat. Physiology & Behavior, 135, 1–6. https://doi.org/10.1016/j.physbeh.2014.05.038.
Rider, D. P., & Kametani, N. N. (1987). Intermittent reinforcement of a continuous response. Journal of the Experimental Analysis of Behavior, 47, 81–95.
Rudski, J. M., Billington, C. J., & Levine, A. S. (1994). Naloxone’s effects on operant responding depend upon level of deprivation. Pharmacology Biochemistry and Behavior, 49, 377–383. https://doi.org/10.1016/0091-3057(94)90437-5.
Schneider, M., Heise, V., & Spanagel, R. (2010). Differential involvement of the opioid receptor antagonist naloxone in motivational and hedonic aspects of reward. Behavioural Brain Research, 208, 466–472. https://doi.org/10.1016/j.bbr.2009.12.013.
Schulze, G. E., & Paule, M. G. (1991). Effects of morphine sulfate on operant behavior in rhesus monkeys. Pharmacology Biochemistry and Behavior, 38, 77–83. https://doi.org/10.1016/0091-3057(91)90592-P.
Senkowski, P. C., Vogel, V. A., & Pozulp, N. C. (1978). Differential reinforcement of lever-press durations: Effects of deprivation level and reward magnitude. Learning and Motivation, 9, 446–465. https://doi.org/10.1016/0023-9690(78)90005-x.
Sheppard, B. A., Gross, S. C., Pavelka, S. A., Hall, M. J., & Palmatier, M. I. (2012). Caffeine increases the motivation to obtain non-drug reinforcers in rats. Drug and Alcohol Dependence, 212, 216–222. https://doi.org/10.1016/j.drugalcdep.2012.01.008.
Snyder, S. H., Katims, J. J., Annau, Z., Bruns, R. F., & Daly, J. W. (1981). Adenosine receptors and behavioral actions of methylxanthines. Proceedings of the National Academy of Sciences of the United States of America, 78, 3260–3264.
Stafford, D., & Branch, M. N. (1998). Effects of step size and break-point criterion on progressive-ratio performance. Journal of the Experimental Analysis of Behavior, 70, 123–128. https://doi.org/10.1901/jeab.1998.70-123.
Stafford, D., LeSage, M. G., & Glowa, J. R. (1999). Effects of phentermine on responding maintained by progressive-ratio schedules of cocaine and food delivery in rhesus monkeys. Behavioral Pharmacology, 10, 775–784. https://doi.org/10.1097/00008877-199912000-00009.
Stevenson, J. G., & Clayton, F. L. (1970). A response duration schedule: Effects of training, extinction, and deprivation. Journal of the Experimental Analysis of Behavior, 13, 359–367.
Woinciki, F. H. E., Roberts, D. C. S., & Corwin, R. L. W. (2006). Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type in non-food deprived rats. Pharmacology Biochemistry and Behavior, 84, 197–206. https://doi.org/10.1016/j.pbb.2006.04.015.
Acknowledgements
We are grateful to three anonymous reviewers who provided valuable suggestions on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study was conducted in partial fulfillment of the requirements for Taylor Manning’s senior thesis at the Massachusetts College of Liberal Arts.
Rights and permissions
About this article
Cite this article
Manning, T., Peck, S. & Byrne, T. Effects of Naloxone and Caffeine on Responding under a Progressive-Duration Schedule of Food Delivery. Psychol Rec 68, 39–48 (2018). https://doi.org/10.1007/s40732-018-0265-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40732-018-0265-4