Abstract
Purpose of Review
The purpose of this review is to briefly outline the current state of hemorrhage control and resuscitation in trauma patients with a specific focus on the role viscoelastic assays have in this complex management to include indications for use across all phases of care in the injured patient.
Recent Findings
Viscoelastic assay use to guide blood-product resuscitation in bleeding trauma patients can reduce mortality by up to 50%. Viscoelastic assays also reduce total blood products transfused, reduce ICU length of stay, and reduce costs. There are a large number of observational and retrospective studies evaluating viscoelastic assay use in the initial trauma resuscitation, but only one randomized control trial. There is a paucity of data evaluating use of viscoelastic assays in the operating room, post-operatively, and during ICU management in trauma patients, rendering their use in these settings extrapolative/speculative based on theory and data from other surgical disciplines and settings.
Summary
Both hypocoagulable and hypercoagulable states exist in trauma patients, and viscoelastic assays are better at diagnosing both relative to standard coagulation testing and can better indicate what therapy may be most appropriate. Further study is needed, particularly in the operating room and post-operative/ICU settings in trauma patients.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Centers for Disease Control NCfIPaC. Web-based Injury Statistics Query and Reporting System (WISQARS) [online] Accessed February 14, 2017. [Available from: https://www.cdc.gov/injury/wisqars/leadingcauses.html.
Sobrino J, Shafi S. Timing and causes of death after injuries. Proc (Bayl Univ Med Cent). 2013;26(2):120–3.
Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38(2):185–93.
Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11.
Maegele M, Paffrath T, Bouillon B. Acute traumatic coagulopathy in severe injury: incidence, risk stratification, and treatment options. Dtsch Arztebl Int. 2011;108(49):827–35.
Gonzalez E, Moore EE, Moore HB. Management of trauma-induced coagulopathy with thrombelastography. Crit Care Clin. 2017;33(1):119–34.
•• Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–9 This manuscript reports outcomes from a randomized controlled trial of trauma patients undergoing massive transfusion, demonstrating a 50% reduction in 28-day mortality using thromboelastography to guide massive transfusion in bleeding trauma patients relative to conventional coagulation assays (INR, platelet count, fibrinogen level).
Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–30.
• Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222(4):347–55 This was a large retrospective study of trauma patients that confirmed and clearly defined the U-shaped mortality curve associated with the spectrum of fibrinolysis (patho)phenotypes previously described an earlier and smaller report by the same authors’, demonstrating an increased risk of death when trauma patients had too little fibrinolysis (fibrinolysis shutdown) or too much fibrinolysis (hyperfibrinolysis). This work has formed the basis for many of the current arguments behind selective use of TXA in trauma patients.
Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–7 discussion 7.
Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma--a unified approach. J Trauma. 1982;22(8):672–9.
Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care. 2005;11(6):590–7.
Gando S, Nanzaki S, Morimoto Y, Kobayashi S, Kemmotsu O. Systemic activation of tissue-factor dependent coagulation pathway in evolving acute respiratory distress syndrome in patients with trauma and sepsis. J Trauma. 1999;47(4):719–23.
Park MS, Martini WZ, Dubick MA, Salinas J, Butenas S, Kheirabadi BS, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma. 2009;67(2):266–75 discussion 75-6.
Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma. 1997;42(4):716–20 discussion 20-2.
Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80(1):16–23 discussion −5.
Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21.
Moore EE, Moore HB, Gonzalez E, Chapman MP, Hansen KC, Sauaia A, et al. Postinjury fibrinolysis shutdown: rationale for selective tranexamic acid. J Trauma Acute Care Surg. 2015;78(6 Suppl 1):S65–9.
Moore HB, Moore EE, Chapman MP, Hansen KC, Cohen MJ, Pieracci FM, et al. Does tranexamic acid improve clot strength in severely injured patients who have elevated fibrin degradation products and low fibrinolytic activity, measured by thrombelastography? J Am Coll Surg. 2019;229(1):92–101.
McCully BH, Connelly CR, Fair KA, Holcomb JB, Fox EE, Wade CE, et al. Onset of coagulation function recovery is delayed in severely injured trauma patients with venous thromboembolism. J Am Coll Surg. 2017;225(1):42–51.
Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, et al. Timing of pharmacologic venous thromboembolism prophylaxis in severe traumatic brain injury: a propensity-matched cohort study. J Am Coll Surg. 2016;223(4):621–31 e5.
Joseph B, Pandit V, Harrison C, Lubin D, Kulvatunyou N, Zangbar B, et al. Early thromboembolic prophylaxis in patients with blunt solid abdominal organ injuries undergoing nonoperative management: is it safe? Am J Surg. 2015;209(1):194–8.
Rostas JW, Manley J, Gonzalez RP, Brevard SB, Ahmed N, Frotan MA, et al. The safety of low molecular-weight heparin after blunt liver and spleen injuries. Am J Surg. 2015;210(1):31–4.
Murphy PB, Sothilingam N, Charyk Stewart T, Batey B, Moffat B, Gray DK, et al. Very early initiation of chemical venous thromboembolism prophylaxis after blunt solid organ injury is safe. Can J Surg. 2016;59(2):118–22.
Asakura H, Ontachi Y, Mizutani T, Kato M, Saito M, Kumabashiri I, et al. An enhanced fibrinolysis prevents the development of multiple organ failure in disseminated intravascular coagulation in spite of much activation of blood coagulation. Crit Care Med. 2001;29(6):1164–8.
Hardaway RM, Harke H, Tyroch AH, Williams CH, Vazquez Y, Krause GF. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg. 2001;67(4):377–82.
Tanaka T, Tsujinaka T, Kambayashi J, Higashiyama M, Yokota M, Sakon M, et al. The effect of heparin on multiple organ failure and disseminated intravascular coagulation in a sepsis model. Thromb Res. 1990;60(4):321–30.
Richey SL. Tourniquets for the control of traumatic hemorrhage: a review of the literature. World J Emerg Surg. 2007;2:28.
Smith ER, Shapiro GL. Totally tourniquets. The facts & details about different types of tourniquets. JEMS. 2013;38(11):48 50, 2.
King DR, van der Wilden G, Kragh JF Jr, Blackbourne LH. Forward assessment of 79 prehospital battlefield tourniquets used in the current war. J Spec Oper Med. 2012;12(4):33–8.
Lee C, Porter KM, Hodgetts TJ. Tourniquet use in the civilian prehospital setting. Emerg Med J. 2007;24(8):584–7.
Ribeiro Junior MAF, Feng CYD, Nguyen ATM, Rodrigues VC, Bechara GEK, de-Moura RR, et al. The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World J Emerg Surg. 2018;13:20.
•• Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315–26 This was a 501 patient randomized controlled trial evaluating the use of fresh frozen plasma (FFP) versus standard crystalloid-based resuscitation during air medical transport of trauma patients at high risk for ongoing hemorrhage. The authors found significantly reduced 30-day mortality in the group treated with FFP, and importantly, a survival difference was noted as early as 3 h after randomization. The FFP group also had lower INR values upon arrival to the trauma center. This study population had notably longer pre-hospital transport times (median 39 min) relative to the COMBAT study (less than 20 min), which did not find a survival benefit from FFP when compared to standard crystalloid-based resuscitation.
collaborators C-t, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32.
Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36(7 Suppl):S267–74.
Lewin I, Weil MH, Shubin H, Sherwin R. Pulmonary failure associated with clinical shock states. J Trauma. 1971;11(1):22–35.
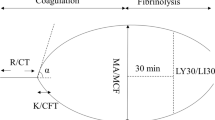
• EMH G, Moore EE, editors. Trauma induced coagulopathy. New York: Springer Scientific; 2016. This is the first textbook dedicated to comprehensively reviewing trauma induced coagulopathy as well as the underlying principles behind hemostasis, coagulation, and fibrinolysis in trauma. This text includes expansive sections explaining the TEG and ROTEM mechanisms as well as interpretation of the output variables.
Ball CG. Damage control resuscitation: history, theory and technique. Can J Surg. 2014;57(1):55–60.
Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the randomized clinical trial. JAMA. 2015;313(5):471–82.
Sheppard FR, Schaub LJ, Cap AP, Macko AR, Moore HB, Moore EE, et al. Whole blood mitigates the acute coagulopathy of trauma and avoids the coagulopathy of crystalloid resuscitation. J Trauma Acute Care Surg. 2018;85(6):1055–62.
MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44.
Cannon WBFJ, Cowell E. The preventive treatment of wound shock. JAMA. 1918;70:618–21.
Moore HB, Moore EE, Liras IN, Wade C, Huebner BR, Burlew CC, et al. Targeting resuscitation to normalization of coagulating status: hyper and hypocoagulability after severe injury are both associated with increased mortality. Am J Surg. 2017;214(6):1041–5.
Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240(3):490–6 discussion 6–8.
Martini WZ. Coagulation complications following trauma. Mil Med Res. 2016;3:35.
O’Malley KF, Ross SE. Pulmonary embolism in major trauma patients. J Trauma. 1990;30(6):748–50.
Subramanian M, Kaplan LJ, Cannon JW. Thromboelastography-guided resuscitation of the trauma patient. JAMA Surg. 2019.
Gale AJ. Continuing education course #2: current understanding of hemostasis. Toxicol Pathol. 2011;39(1):273–80.
Eisenberg JM, Clarke JR, Sussman SA. Prothrombin and partial thromboplastin times as preoperative screening tests. Arch Surg. 1982;117(1):48–51.
Hartert H., Klin Wochenschr. 1948;26(37–38):577–583 [Not Available].
De Nicola P, Mazzetti GM. Evaluation of thrombelastography. Am J Clin Pathol. 1955;23(4):447–52.
Von Kaulla KN, Weiner M. Studies of coagulation and fibrinolysis by new technic of continuous recording. Blood. 1955;10(4):362–9.
Sankarankutty A, Nascimento B, Teodoro da Luz L, Rizoli S. TEG(R) and ROTEM(R) in trauma: similar test but different results? World J Emerg Surg. 2012;7(Suppl 1):S3.
Harr JN, Moore EE, Ghasabyan A, Chin TL, Sauaia A, Banerjee A, et al. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013;39(1):45–9.
Dias JD, Haney EI, Mathew BA, Lopez-Espina CG, Orr AW, Popovsky MA. New-generation thromboelastography: comprehensive evaluation of citrated and heparinized blood sample storage effect on clot-forming variables. Arch Pathol Lab Med. 2017;141(4):569–77.
Meledeo MA, Peltier GC, McIntosh CS, Voelker CR, Bynum JA, Cap AP. Functional stability of the TEG 6 s hemostasis analyzer under stress. J Trauma Acute Care Surg. 2018;84(6S Suppl 1):S83–S8.
•• Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, et al. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. J Trauma Acute Care Surg. 2017;82(1):114–9 R-TEG® data was analyzed from 190 trauma patients to determine the predictive power of ACT, angle, MA, and LY30 in relation to outcomes including whether massive transfusion was required, 24-h mortality, ICU-, and ventilator-free days. ROC curves were generated and guided the authors to suggest the values at which the different R-TEG® variables reasonably suggested the need for massive transfusion as well as R-TEG® variable cutoffs for individual component transfusions. Of the R-TEG® variables, angle and MA were found to be the strongest predictors of requiring massive transfusion (AUROC ≥ 80%).
Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ Jr, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378–85 discussion 85–6.
Fahrendorff M, Oliveri RS, Johansson PI. The use of viscoelastic haemostatic assays in goal-directing treatment with allogeneic blood products - a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med. 2017;25(1):39.
Mohamed M, Majeske K, Sachwani GR, Kennedy K, Salib M, McCann M. The impact of early thromboelastography directed therapy in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2017;25(1):99.
Yeung MC, Tong SY, Tong PY, Cheung BH, Ng JY, Leung GK. Use of viscoelastic haemostatic assay in emergency and elective surgery. Hong Kong Med J. 2015;21(1):45–51.
Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomized trial. Lancet. 2018;392(10144):283–91.
Schroll R, Swift D, Tatum D, Couch S, Heaney JB, Llado-Farrulla M, et al. Accuracy of shock index versus ABC score to predict need for massive transfusion in trauma patients. Injury. 2018;49(1):15–9.
Zhu CS, Cobb D, Jonas RB, Pokorny D, Rani M, Cotner-Pouncy T, et al. Shock index and pulse pressure as triggers for massive transfusion. J Trauma Acute Care Surg. 2019.
Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205(4):541–5.
Rozycki GS, Ochsner MG, Schmidt JA, Frankel HL, Davis TP, Wang D, et al. A prospective study of surgeon-performed ultrasound as the primary adjuvant modality for injured patient assessment. J Trauma. 1995;39(3):492–8 discussion 8–500.
Rozycki GS, Ballard RB, Feliciano DV, Schmidt JA, Pennington SD. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg. 1998;228(4):557–67.
McLaughlin DF, Niles SE, Salinas J, Perkins JG, Cox ED, Wade CE, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):S57–63 discussion S.
Callcut RA, Johannigman JA, Kadon KS, Hanseman DJ, Robinson BR. All massive transfusion criteria are not created equal: defining the predictive value of individual transfusion triggers to better determine who benefits from blood. J Trauma. 2011;70(4):794–801.
Zhang J, Jiang R, Liu L, Watkins T, Zhang F, Dong JF. Traumatic brain injury-associated coagulopathy. J Neurotrauma. 2012;29(19):2597–605.
Maegele M. Coagulopathy after traumatic brain injury: incidence, pathogenesis, and treatment options. Transfusion. 2013;53(Suppl 1):28S–37S.
Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70(6):1334–45.
Gennarelli TA, Champion HR, Copes WS, Sacco WJ. Comparison of mortality, morbidity, and severity of 59,713 head injured patients with 114,447 patients with extracranial injuries. J Trauma. 1994;37(6):962–8.
McMahon CG, Yates DW, Campbell FM, Hollis S, Woodford M. Unexpected contribution of moderate traumatic brain injury to death after major trauma. J Trauma. 1999;47(5):891–5.
van der Jagt M. Fluid management of the neurological patient: a concise review. Crit Care. 2016;20(1):126.
Kvint S, Schuster J, Kumar MA. Neurosurgical applications of viscoelastic hemostatic assays. Neurosurg Focus. 2017;43(5):E9.
Foreman PM, Harrigan MR. Blunt traumatic extracranial cerebrovascular injury and ischemic stroke. Cerebrovasc Dis Extra. 2017;7(1):72–83.
Shafafy R, Suresh S, Afolayan JO, Vaccaro AR, Panchmatia JR. Blunt vertebral vascular injury in trauma patients: ATLS((R)) recommendations and review of current evidence. J Spine Surg. 2017;3(2):217–25.
Dewan MC, Ravindra VM, Gannon S, Prather CT, Yang GL, Jordan LC, et al. Treatment practices and outcomes after blunt cerebrovascular injury in children. Neurosurgery. 2016;79(6):872–8.
Brommeland T, Helseth E, Aarhus M, Moen KG, Dyrskog S, Bergholt B, et al. Best practice guidelines for blunt cerebrovascular injury (BCVI). Scand J Trauma Resusc Emerg Med. 2018;26(1):90.
Sumislawski JJ, Moore HB, Moore EE, Swope ML, Pieracci FM, Fox CJ, et al. Not all in your head (and neck): stroke after blunt cerebrovascular injury is associated with systemic hypercoagulability. J Trauma Acute Care Surg. 2019;87(5):1082–7.
Asensio JA, Roldan G, Petrone P, Rojo E, Tillou A, Kuncir E, et al. Operative management and outcomes in 103 AAST-OIS grades IV and V complex hepatic injuries: trauma surgeons still need to operate, but angioembolization helps. J Trauma. 2003;54(4):647–53 discussion 53–4.
Doklestic K, Stefanovic B, Gregoric P, Ivancevic N, Loncar Z, Jovanovic B, et al. Surgical management of AAST grades III-V hepatic trauma by damage control surgery with perihepatic packing and definitive hepatic repair-single centre experience. World J Emerg Surg. 2015;10:34.
Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515–23.
Raum D, Marcus D, Alper CA, Levey R, Taylor PD, Starzl TE. Synthesis of human plasminogen by the liver. Science. 1980;208(4447):1036–7.
Walker FJ. Protein C deficiency in liver disease. Ann Clin Lab Sci. 1990;20(2):106–12.
Harrison MF. The misunderstood coagulopathy of liver disease: a review for the acute setting. West J Emerg Med. 2018;19(5):863–71.
Davis JPE, Northup PG, Caldwell SH, Intagliata NM. Viscoelastic testing in liver disease. Ann Hepatol. 2018;17(2):205–13.
Georgiou C, Inaba K, Teixeira PG, Hadjizacharia P, Chan LS, Brown C, et al. Cirrhosis and trauma are a lethal combination. World J Surg. 2009;33(5):1087–92.
Christmas AB, Wilson AK, Franklin GA, Miller FB, Richardson JD, Rodriguez JL. Cirrhosis and trauma: a deadly duo. Am Surg. 2005;71(14):996–1000.
Demetriades D, Constantinou C, Salim A, Velmahos G, Rhee P, Chan L. Liver cirrhosis in patients undergoing laparotomy for trauma: effect on outcomes. J Am Coll Surg. 2004;199(4):538–42.
Wahlstrom K, Ney AL, Jacobson S, Odland MD, Van Camp JM, Rodriguez JL, et al. Trauma in cirrhotics: survival and hospital sequelae in patients requiring abdominal exploration. Am Surg. 2000;66(13):1071–6.
Boufous S, Finch C, Lord S, Close J. The increasing burden of pelvic fractures in older people, New South Wales. Australia Injury. 2005;36(13):1323–9.
Cryer HM, Miller FB, Evers BM, Rouben LR, Seligson DL. Pelvic fracture classification: correlation with hemorrhage. J Trauma. 1988;28(7):973–80.
Rothenberger D, Velasco R, Strate R, Fischer RP, Perry JF Jr. Open pelvic fracture: a lethal injury. J Trauma. 1978;18(3):184–7.
Henry SM, Pollak AN, Jones AL, Boswell S, Scalea TM. Pelvic fracture in geriatric patients: a distinct clinical entity. J Trauma. 2002;53(1):15–20.
Ben-Menachem Y, Coldwell DM, Young JW, Burgess AR. Hemorrhage associated with pelvic fractures: causes, diagnosis, and emergent management. AJR Am J Roentgenol. 1991;157(5):1005–14.
Coccolini F, Stahel PF, Montori G, Biffl W, Horer TM, Catena F, et al. Pelvic trauma: WSES classification and guidelines. World J Emerg Surg. 2017;12:5.
Colson PH, Gaudard P, Fellahi JL, Bertet H, Faucanie M, Amour J, et al. Active bleeding after cardiac surgery: a prospective observational multicenter study. PLoS One. 2016;11(10):e0162396.
Shen L, Tabaie S, Ivascu N. Viscoelastic testing inside and beyond the operating room. J Thorac Dis. 2017;9(Suppl 4):S299–308.
Curry NS, Davenport R, Pavord S, Mallett SV, Kitchen D, Klein AA, et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Hematology Guideline. Br J Haematol. 2018;182(6):789–806.
Erdoes G, Schloer H, Eberle B, Nagler M. Next generation viscoelasticity assays in cardiothoracic surgery: feasibility of the TEG6s system. PLoS One. 2018;13(14):e0209360.
Roullet S, de Maistre E, Ickx B, Blais N, Susen S, Faraoni D, et al. Position of the French Working Group on Perioperative Hemostasis (GIHP) on viscoelastic tests: what role for which indication in bleeding situations? Anaesth Crit Care Pain Med. 2019;38(5):539–48.
Alamo JM, Leon A, Mellado P, Bernal C, Marin LM, Cepeda C, et al. Is “intra-operating room” thromboelastometry useful in liver transplantation? A case-control study in 303 patients. Transplant Proc. 2013;45(12):3637–9.
Wang YX, Griffith JF, Deng M, Heather TM, Zhang YF, Yan SX, et al. Compromised perfusion in femoral head in normal rats: distinctive perfusion MRI evidence of contrast washout delay. Br J Radiol. 2012;85(1016):e436–41.
Leon-Justel A, Noval-Padillo JA, Alvarez-Rios AI, Mellado P, Gomez-Bravo MA, Alamo JM, et al. Point-of-care hemostasis monitoring during liver transplantation reduces transfusion requirements and improves patient outcome. Clin Chim Acta. 2015;446:277–83.
Gurusamy KS, Pissanou T, Pikhart H, Vaughan J, Burroughs AK, Davidson BR. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011;(14):CD009052.
McCrossin KE, Bramley DE, Hessian E, Hutcheon E, Imberger G. Viscoelastic testing for hepatic surgery: a systematic review with meta-analysis-a protocol. Syst Rev. 2016;5(1):151.
Spinella PC, Pidcoke HF, Strandenes G, Hervig T, Fisher A, Jenkins D, et al. Whole blood for hemostatic resuscitation of major bleeding. Transfusion. 2016;56(Suppl 2):S190–202.
Spinella PC, Cap AP. Whole blood: back to the future. Curr Opin Hematol. 2016;23(6):536–42.
Yazer MH, Jackson B, Sperry JL, Alarcon L, Triulzi DJ, Murdock AD. Initial safety and feasibility of cold-stored uncrossmatched whole blood transfusion in civilian trauma patients. J Trauma Acute Care Surg. 2016;81(1):21–6.
Neal MD, Moore HB, Moore EE, Freeman K, Cohen MJ, Sperry JL, et al. Clinical assessment of trauma-induced coagulopathy and its contribution to postinjury mortality: a TACTIC proposal. J Trauma Acute Care Surg. 2015;79(3):490–2.
Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(26):1601–6.
Park MS, Perkins SE, Spears GM, Ashrani AA, Leibson CL, Boos CM, et al. Risk factors for venous thromboembolism after acute trauma: a population-based case-cohort study. Thromb Res. 2016;144:40–5.
Frohlich M, Lefering R, Probst C, Paffrath T, Schneider MM, Maegele M, et al. Epidemiology and risk factors of multiple-organ failure after multiple trauma: an analysis of 31,154 patients from the Trauma Register DGU. J Trauma Acute Care Surg. 2014;76(4):921–7 discussion 7–8.
Kuo SCH, Kuo PJ, Chen YC, Chien PC, Hsieh HY, Hsieh CH. Comparison of the new Exponential Injury Severity Score with the Injury Severity Score and the New Injury Severity Score in trauma patients: a cross-sectional study. PLoS One. 2017;12(13):e0187871.
Blasi A, Molina V, Sanchez-Cabus S, Balust J, Garcia-Valdecasas JC, Taura P. Prediction of thromboembolic complications after liver resection for cholangiocarcinoma: is there a place for thromboelastometry? Blood Coagul Fibrinolysis. 2018;29(1):61–6.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Dr. H. Moore reports grants from the National Institutes of Health during the conduct of the study, another from Thrombo Therapeutics, Inc. outside the submitted work. In addition, Dr. Moore has a patent pending A Modified Coagulation Assay That Rapidly Unmasks Pathological Fibrinolysis Phenotypes in a Wide Spectrum of Human Diseases. In addition, Dr. Moore has a patent Fibrinolysis Detection issued.
Dr. Ernest Moore reports non-financial, research support from Haemonetics, non-financial support from Instrumentation Laboratory, and non-financial support from Stago outside the submitted work. In addition, Dr. E. Moore has a patent Fibrinolysis Detection issued and is Co-Founder of ThromboTherapeutics.
Dr. Barrett reports grants from National Institutes of Health during the conduct of the study and another from Thrombo Therapeutics, Inc. outside the submitted work. In addition, Dr. Barrett has a patent pending A Modified Coagulation Assay That Rapidly Unmasks Pathological Fibrinolysis Phenotypes in a Wide Spectrum of Human Diseases.
Dr. Yaffe reports grants from National Institutes of Health during the conduct of the study and another from Thrombo Therapeutics, Inc. outside the submitted work. In addition, Dr. Barrett has a patent pending A Modified Coagulation Assay That Rapidly Unmasks Pathological Fibrinolysis Phenotypes in a Wide Spectrum of Human Diseases.
Mr. Dhara has nothing to disclose.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors were performed in accordance with all applicable ethical standards including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hemostasis after Trauma
Rights and permissions
About this article
Cite this article
Dhara, S., Moore, E.E., Yaffe, M.B. et al. Modern Management of Bleeding, Clotting, and Coagulopathy in Trauma Patients: What Is the Role of Viscoelastic Assays?. Curr Trauma Rep 6, 69–81 (2020). https://doi.org/10.1007/s40719-020-00183-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40719-020-00183-w