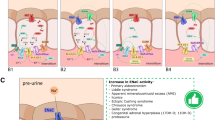
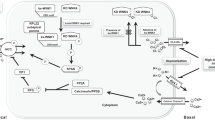
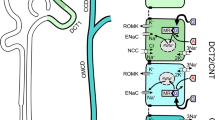
Abstract
In recent years, our understanding of the physiology of the aldosterone-sensitive distal nephron (ASDN) has greatly advanced thanks to the discovery of the complex with-no-lysine kinase (WNK) signaling and the molecular characterization of the epithelial sodium channel (ENaC). A series of studies, initially focused on rare tubulopathies such as Gordon and Liddle syndromes, eventually led to a partial elucidation of the so-called “aldosterone paradox”, the traditional explanation of the physiology of such disparate conditions such as hyperkalemia and low effective arterial blood volume. The physiology of the ASDN is herein illustrated in light of the novel acquisitions in an easy-to-understand fashion, with the aim of giving the practicing nephrologist a solid “first glance” into this exciting but challenging field. Focus is on ion channels and transporters, their regulation by key hormones such as aldosterone and angiotensin II, and dietary implications.






Similar content being viewed by others
Abbreviations
- ENaC:
-
Epithelial sodium channel
- WNK:
-
With no lysine kinase
- ASDN:
-
Aldosterone-sensitive distal nephron
- AII:
-
Angiotensin II
- DCT:
-
Distal convoluted tubule
- CNT:
-
Connecting tubule
- CD:
-
Collecting duct
- DCT2:
-
The late portion of the distal convoluted tubule
- CCD:
-
Cortical collecting duct
- MR:
-
Mineralocorticoid receptor
- 11BHSD2:
-
11β-Hydroxysteroid dehydrogenase type 2
- DCT1:
-
The early portion of the distal convoluted tubule
- NCC:
-
Sodium chloride cotransporter
- NDCBE:
-
Sodium-driven chloride bicarbonate exchanger
- SPAK:
-
Ste20-like proline–alanine rich kinase
- OSR1:
-
Oxidative stress responsive kinase 1
- KLHL3:
-
Kelch-like 3
- CUL3:
-
Cullin 3
- Nedd4-2:
-
Neural precursor cell expressed developmentally down-regulated protein 4-2
- ROMK:
-
Renal outer medullary potassium channel
- BK:
-
Big potassium channels
- Kir4.1/5.1:
-
Inward-rectifier potassium channel 4.1/5.1
- CLCNKB:
-
Chloride channel, kidney b
- AT1R:
-
Angiotensin II receptor type 1
- SGK-1:
-
Serum and glucocorticoid-regulated kinase 1
- FIKS:
-
Flow-induced potassium secretion
- BS:
-
Bartter syndrome
- GS:
-
Gitelman syndrome
- TAL:
-
Thick ascending limb of the loop of Henle
- NKCC2:
-
Sodium–potassium–chloride cotransporter 2
- CaSR:
-
Calcium-sensing receptor
- TRPV5:
-
Transient receptor potential cation channel subfamily V
References
Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC (1994) Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367(6462):463–467. https://doi.org/10.1038/367463a0
Canessa CM, Horisberger JD, Rossier BC (1993) Epithelial sodium channel related to proteins involved in neurodegeneration. Nature 361(6411):467–470. https://doi.org/10.1038/361467a0
Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P (1993) Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett 318(1):95–99
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science 293(5532):1107–1112. https://doi.org/10.1126/science.1062844
Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G (2011) Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda) 26(2):115–123. https://doi.org/10.1152/physiol.00049.2010
Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, Ellison DH (1998) 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na–Cl cotransporter expression by distal tubules. J Am Soc Nephrol 9(8):1347–1358
Funder JW (2013) Mineralocorticoid receptor antagonists: emerging roles in cardiovascular medicine. Integr Blood Press Control 6:129–138. https://doi.org/10.2147/IBPC.S13783
Palmer LG, Schnermann J (2015) Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 10(4):676–687. https://doi.org/10.2215/CJN.12391213
Subramanya AR, Ellison DH (2014) Distal convoluted tubule. Clin J Am Soc Nephrol 9(12):2147–2163. https://doi.org/10.2215/CJN.05920613
Roy A, Al-bataineh MM, Pastor-Soler NM (2015) Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol 10(2):305–324. https://doi.org/10.2215/CJN.08880914
Chen JC, Lo YF, Lin YW, Lin SH, Huang CL, Cheng CJ (2019) WNK4 kinase is a physiological intracellular chloride sensor. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1817220116
Welling PA (2016) Roles and regulation of renal K channels. Annu Rev Physiol 78:415–435. https://doi.org/10.1146/annurev-physiol-021115-105423
Hadchouel J, Ellison DH, Gamba G (2016) Regulation of renal electrolyte transport by WNK and SPAK–OSR1 kinases. Annu Rev Physiol 78:367–389. https://doi.org/10.1146/annurev-physiol-021115-105431
Shibata S, Arroyo JP, Castaneda-Bueno M, Puthumana J, Zhang J, Uchida S, Stone KL, Lam TT, Lifton RP (2014) Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci USA 111(43):15556–15561. https://doi.org/10.1073/pnas.1418342111
Ferdaus MZ, Mukherjee A, Nelson JW, Blatt PJ, Miller LN, Terker A, Staub O, Lin DH, McCormick JA (2019) Mg2+ restriction downregulates NCC through NEDD4-2 and prevents its activation by hypokalemia. Am J Physiol Ren Physiol. https://doi.org/10.1152/ajprenal.00216.2019
Williams CR, Mistry M, Cheriyan AM, Williams JM, Naraine MK, Ellis CL, Mallick R, Mistry AC, Gooch JL, Ko B, Cai H, Hoover RS (2019) Zinc deficiency induces hypertension by promoting renal Na+ reabsorption. Am J Physiol Ren Physiol 316(4):F646–F653. https://doi.org/10.1152/ajprenal.00487.2018
Chambrey R, Trepiccione F (2015) Relative roles of principal and intercalated cells in the regulation of sodium balance and blood pressure. Curr Hypertens Rep 17(4):538. https://doi.org/10.1007/s11906-015-0538-0
Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D (2010) The Na+-dependent chloride–bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Investig 120(5):1627–1635. https://doi.org/10.1172/JCI40145
Eladari D, Chambrey R, Peti-Peterdi J (2012) A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74:325–349. https://doi.org/10.1146/annurev-physiol-020911-153225
Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW (2004) NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl-conservation. Hypertension 44(6):982–987. https://doi.org/10.1161/01.HYP.0000145863.96091.89
Mironova E, Bugaj V, Roos KP, Kohan DE, Stockand JD (2012) Aldosterone-independent regulation of the epithelial Na+ channel (ENaC) by vasopressin in adrenalectomized mice. Proc Natl Acad Sci USA 109(25):10095–10100. https://doi.org/10.1073/pnas.1201978109
Raff H (1987) Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol Regul Integr Comp Physiol 252(4 Pt 2):R635–R644. https://doi.org/10.1152/ajpregu.1987.252.4.R635
Hou J (2016) Paracellular transport in the collecting duct. Curr Opin Nephrol Hypertens 25(5):424–428. https://doi.org/10.1097/MNH.0000000000000253
Gong Y, Yu M, Yang J, Gonzales E, Perez R, Hou M, Tripathi P, Hering-Smith KS, Hamm LL, Hou J (2014) The Cap1–claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc Natl Acad Sci USA 111(36):E3766–E3774. https://doi.org/10.1073/pnas.1406741111
Gong Y, Wang J, Yang J, Gonzales E, Perez R, Hou J (2015) KLHL3 regulates paracellular chloride transport in the kidney by ubiquitination of claudin-8. Proc Natl Acad Sci USA 112(14):4340–4345. https://doi.org/10.1073/pnas.1421441112
Palmer BF (2015) Regulation of potassium homeostasis. Clin J Am Soc Nephrol 10(6):1050–1060. https://doi.org/10.2215/CJN.08580813
Su XT, Ellison DH, Wang WH (2019) Kir4.1/Kir5.1 in the DCT plays a role in the regulation of renal K+ excretion. Am J Physiol Ren Physiol 316(3):F582–F586. https://doi.org/10.1152/ajprenal.00412.2018
Duan XP, Gu L, Xiao Y, Gao ZX, Wu P, Zhang YH, Meng XX, Wang JL, Zhang DD, Lin DH, Wang WH, Gu R (2019) Norepinephrine-induced stimulation of Kir4.1/Kir5.1 is required for the activation of NaCl transporter in distal convoluted tubule. Hypertension 73(1):112–120. https://doi.org/10.1161/hypertensionaha.118.11621
Kamel KS, Schreiber M, Halperin ML (2018) Renal potassium physiology: integration of the renal response to dietary potassium depletion. Kidney Int 93(1):41–53. https://doi.org/10.1016/j.kint.2017.08.018
Huang CL, Kuo E (2007) Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol 18(10):2649–2652. https://doi.org/10.1681/ASN.2007070792
Carrisoza-Gaytan R, Carattino MD, Kleyman TR, Satlin LM (2016) An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron. Am J Physiol Cell Physiol 310(4):C243–C259. https://doi.org/10.1152/ajpcell.00328.2015
Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F (2001) Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Ren Physiol 280(4):F675–F682. https://doi.org/10.1152/ajprenal.2001.280.4.F675
Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP (2007) An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA 104(10):4025–4029. https://doi.org/10.1073/pnas.0611728104
Alvarez de la Rosa D, Zhang P, Naray-Fejes-Toth A, Fejes-Toth G, Canessa CM (1999) The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes. J Biol Chem 274(53):37834–37839
de la Alvarez Rosa D, Gimenez I, Forbush B, Canessa CM (2006) SGK1 activates Na+–K+-ATPase in amphibian renal epithelial cells. Am J Physiol Cell Physiol 290(2):C492–C498. https://doi.org/10.1152/ajpcell.00556.2004
Palmer LG, Frindt G (2000) Aldosterone and potassium secretion by the cortical collecting duct. Kidney Int 57(4):1324–1328. https://doi.org/10.1046/j.1523-1755.2000.00970.x
Welling PA (2013) Regulation of renal potassium secretion: molecular mechanisms. Semin Nephrol 33(3):215–228. https://doi.org/10.1016/j.semnephrol.2013.04.002
Cheng L, Poulsen SB, Wu Q, Esteva-Font C, Olesen ETB, Peng L, Olde B, Leeb-Lundberg LMF, Pisitkun T, Rieg T, Dimke H, Fenton RA (2019) Rapid aldosterone-mediated signaling in the DCT increases activity of the thiazide-sensitive NaCl cotransporter. J Am Soc Nephrol 30(8):1454–1470. https://doi.org/10.1681/ASN.2018101025
Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, Uchida S, Shibata S (2017) Hypokalemia and pendrin induction by aldosterone. Hypertension 69(5):855–862. https://doi.org/10.1161/HYPERTENSIONAHA.116.08519
Hirohama D, Ayuzawa N, Ueda K, Nishimoto M, Kawarazaki W, Watanabe A, Shimosawa T, Marumo T, Shibata S, Fujita T (2018) Aldosterone is essential for angiotensin II-induced upregulation of pendrin. J Am Soc Nephrol 29(1):57–68. https://doi.org/10.1681/ASN.2017030243
Reilly RF, Peixoto AJ, Desir GV (2010) The evidence-based use of thiazide diuretics in hypertension and nephrolithiasis. Clin J Am Soc Nephrol 5(10):1893–1903. https://doi.org/10.2215/CJN.04670510
Lee CT, Chen HC, Lai LW, Yong KC, Lien YH (2007) Effects of furosemide on renal calcium handling. Am J Physiol Ren Physiol 293(4):F1231–F1237. https://doi.org/10.1152/ajprenal.00038.2007
Bazua-Valenti S, Rojas-Vega L, Castaneda-Bueno M, Barrera-Chimal J, Bautista R, Cervantes-Perez LG, Vazquez N, Plata C, Murillo-de-Ozores AR, Gonzalez-Mariscal L, Ellison DH, Riccardi D, Bobadilla NA, Gamba G (2018) The calcium-sensing receptor increases activity of the renal NCC through the WNK4-SPAK pathway. J Am Soc Nephrol 29(7):1838–1848. https://doi.org/10.1681/ASN.2017111155
Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW (1997) Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Investig 99(6):1399–1405. https://doi.org/10.1172/JCI119299
Sorensen MV, Matos JE, Praetorius HA, Leipziger J (2010) Colonic potassium handling. Pflugers Arch 459(5):645–656. https://doi.org/10.1007/s00424-009-0781-9
Guagliardo NA, Yao J, Hu C, Barrett PQ (2012) Minireview: aldosterone biosynthesis: electrically gated for our protection. Endocrinology 153(8):3579–3586. https://doi.org/10.1210/en.2012-1339
Greenlee M, Wingo CS, McDonough AA, Youn JH, Kone BC (2009) Narrative review: evolving concepts in potassium homeostasis and hypokalemia. Ann Intern Med 150(9):619–625
Preston RA, Afshartous D, Rodco R, Alonso AB, Garg D (2015) Evidence for a gastrointestinal–renal kaliuretic signaling axis in humans. Kidney Int 88(6):1383–1391. https://doi.org/10.1038/ki.2015.243
Shafiee MA, Charest AF, Cheema-Dhadli S, Glick DN, Napolova O, Roozbeh J, Semenova E, Sharman A, Halperin ML (2005) Defining conditions that lead to the retention of water: the importance of the arterial sodium concentration. Kidney Int 67(2):613–621. https://doi.org/10.1111/j.1523-1755.2005.67117.x
Hoorn EJ, Zietse R (2015) Gut–kidney kaliuretic signaling: looking forward to feeding. Kidney Int 88(6):1230–1232. https://doi.org/10.1038/ki.2015.272
Palmer LG, Antonian L, Frindt G (1994) Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104(4):693–710. https://doi.org/10.1085/jgp.104.4.693
Stanton B, Pan L, Deetjen H, Guckian V, Giebisch G (1987) Independent effects of aldosterone and potassium on induction of potassium adaptation in rat kidney. J Clin Investig 79(1):198–206. https://doi.org/10.1172/JCI112783
Xue C, Siragy HM (2005) Local renal aldosterone system and its regulation by salt, diabetes, and angiotensin II type 1 receptor. Hypertension 46(3):584–590. https://doi.org/10.1161/01.HYP.0000175814.18550.c0
Kobayashi M, Yasuoka Y, Sato Y, Zhou M, Abe H, Kawahara K, Okamoto H (2011) Upregulation of calbindin D28k in the late distal tubules in the potassium-loaded adrenalectomized mouse kidney. Clin Exp Nephrol 15(3):355–362. https://doi.org/10.1007/s10157-011-0414-4
Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J (2015) Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26(2):425–438. https://doi.org/10.1681/ASN.2013111156
Gumz ML, Rabinowitz L, Wingo CS (2015) An integrated view of potassium homeostasis. N Engl J Med 373(1):60–72. https://doi.org/10.1056/NEJMra1313341
Wu P, Gao ZX, Su XT, Wang MX, Wang WH, Lin DH (2019) Kir4.1/Kir5.1 activity is essential for dietary sodium intake-induced modulation of Na–Cl cotransporter. J Am Soc Nephrol 30(2):216–227. https://doi.org/10.1681/asn.2018080799
Cornelius RJ, Wang B, Wang-France J, Sansom SC (2016) Maintaining K+ balance on the low-Na+, high-K+ diet. Am J Physiol Ren Physiol 310(7):F581–F595. https://doi.org/10.1152/ajprenal.00330.2015
Palmer BF, Clegg DJ (2016) Achieving the benefits of a high-potassium, paleolithic diet, without the toxicity. Mayo Clin Proc 91(4):496–508. https://doi.org/10.1016/j.mayocp.2016.01.012
Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N, DASH Collaborative Research Group (1997) A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 336(16):1117–1124. https://doi.org/10.1056/nejm199704173361601
Hajjar IM, Grim CE, George V, Kotchen TA (2001) Impact of diet on blood pressure and age-related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med 161(4):589–593
Zuckerman JM, Assimos DG (2009) Hypocitraturia: pathophysiology and medical management. Rev Urol 11(3):134–144
Osis G, Webster KL, Harris AN, Lee HW, Chen C, Fang L, Romero MF, Khattri RB, Merritt ME, Verlander JW, Weiner ID (2019) Regulation of renal NaDC1 expression and citrate excretion by NBCe1-A. Am J Physiol Ren Physiol. https://doi.org/10.1152/ajprenal.00015.2019
Acknowledgements
The authors would like to thank Prof. G. Capasso and F. Trepiccione for their useful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with animals or human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rossi, G.M., Regolisti, G., Peyronel, F. et al. Recent insights into sodium and potassium handling by the aldosterone-sensitive distal nephron: a review of the relevant physiology. J Nephrol 33, 431–445 (2020). https://doi.org/10.1007/s40620-019-00684-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-019-00684-1