Abstract
Background
Activation of the farnesoid X receptor (FXR), a member of the nuclear receptor steroid superfamily, leads to anti-inflammatory and anti-fibrotic effects in several tissues, including the lung. We have recently demonstrated a protective effect of the farnesoid X receptor (FXR) agonist obeticholic acid (OCA) in rat models of monocrotaline (MCT)-induced pulmonary arterial hypertension (PAH) and bleomycin-induced pulmonary fibrosis. The aim of the present study was to investigate whether the positive effects of OCA treatment could be exerted also in established MCT-induced PAH, i.e., starting treatment 2 weeks after MCT administration.
Methods
Rats with MCT-induced PAH were treated, 2 weeks after MCT administration, with OCA or tadalafil for two additional weeks. Pulmonary functional tests were performed at week 2 (before treatment) and four (end of treatment). At the same time points, lung morphological features and expression profile of genes related to smooth muscle relaxation/contraction and tissue remodeling were also assessed.
Results
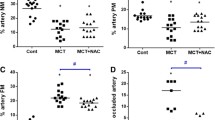
2 weeks after MCT-induced injury, the treadmill resistance (a functional parameter related to pulmonary hypertension) was significantly decreased. At the same time point, we observed right ventricular hypertrophy and vascular remodeling, with upregulation of genes related to inflammation. At week 4, we observed a further worsening of the functional and morphological parameters, accompanied by dysregulation of inflammatory and extracellular matrix markers mRNA expression. Administration of OCA (3 or 10 mg/kg/day), starting 2 weeks after MCT-induced injury, significantly improved pulmonary function, effectively normalizing the exercise capacity. OCA also reverted most of the lung alterations, with a significant reduction of lung vascular wall thickness, right ventricular hypertrophy, and restoration of the local balance between relaxant and contractile pathways. Markers of remodeling pathways were also normalized by OCA treatment. Notably, results with OCA treatment were similar, or even superior, to those obtained with tadalafil, a recently approved treatment for pulmonary hypertension.
Conclusions
The results of this study demonstrate a significant therapeutic effect of OCA in established MCT-induced PAH, improving exercise capacity associated with reduction of right ventricular hypertrophy and lung vascular remodeling. Thus, OCA dosing in a therapeutic protocol restores the balance between relaxant and contractile pathways in the lung, promoting cardiopulmonary protective actions in MCT-induced PAH.









Similar content being viewed by others
References
Li T, Chiang JY (2014) Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev 66:948–983
Copple BL, Li T (2016) Pharmacology of bile acid receptors: evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res 104:9–21
Cariou B, van Harmelen K, Duran-Sandoval D et al (2006) The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem 281:11039–11049
Houten SM, Volle DH, Cummins CL et al (2007) In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol 21:1312–1323
Schote AB, Turner JD, Schiltz J, Muller CP (2007) Nuclear receptors in human immune cells: expression and correlations. Mol Immunol 44:1436–1445
Higashiyama H, Kinoshita M, Asano S (2008) Immunolocalization of farnesoid X receptor (FXR) in mouse tissues using tissue microarray. Acta Histochem 110:86–93
Lefebvre P, Cariou B, Lien F et al (2009) Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89:147–191
Popescu IR, Helleboid-Chapman A, Lucas A et al (2010) The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett 584:2845–2851
Ali AH, Carey EJ, Lindor KD (2015) Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med 3:5
Ye L, Jiang Y, Zuo X (2015) Farnesoid-X-receptor expression in monocrotaline-induced pulmonary arterial hypertension and right heart failure. Biochem Biophys Res Commun 467:164–170
Comeglio P, Filippi S, Sarchielli E et al (2017) Anti-fibrotic effects of chronic treatment with the selective FXR agonist obeticholic acid in the bleomycin-induced rat model of pulmonary fibrosis. J Steroid Biochem Mol Biol 168:26–37
He F, Li J, Mu Y et al (2006) Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res 98:192–199
Hendrick SM, Mroz MS, Greene CM et al (2014) Bile acids stimulate chloride secretion through CFTR and calcium-activated Cl- channels in Calu-3 airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 307:407–418
Vignozzi L, Morelli A, Cellai I et al (2017) Cardiopulmonary protective effects of the selective FXR agonist obeticholic acid in the rat model of monocrotaline-induced pulmonary hypertension. J Steroid Biochem Mol Biol 165:277–292
Shaik FB, Panati K, Narasimha VR, Narala VR (2015) Chenodeoxycholic acid attenuates ovalbumin-induced airway inflammation in murine model of asthma by inhibiting the T(H)2 cytokines. Biochem Biophys Res Commun 463(4):600–605
Zhang L, Li T, Yu D et al (2012) FXR protects lung from lipopolysaccharide-induced acute injury. Mol Endocrinol 26:27–36
Pellicciari R, Fiorucci S, Camaioni E et al (2002) 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem 45:3569–3572
Markham A, Keam SJ (2016) Obeticholic acid: First global approval. Drugs 76(12):1221–1226
Hirschfield GM, Mason A, Luketic V et al (2015) Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 148:751–761
Nevens F, Andreone P, Mazzella G et al (2016) A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 375(7):631–643
Neuschwander-Tetri BA, Loomba R, Sanyal AJ et al (2015) Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385:956–965
Mudaliar S, Henry RR, Sanyal AJ et al (2013) Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145:574–582
Wang XX, Jiang T, Shen Y et al (2010) Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59:2916–2927
Vignozzi L, Morelli A, Filippi S et al (2011) Farnesoid X receptor activation improves erectile function in animal models of metabolic syndrome and diabetes. J Sex Med 8(1):57–77
Adorini L, Pruzanski M, Shapiro D (2012) Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today 17:988–997
Vignozzi L, Filippi S, Comeglio P et al (2014) Nonalcoholic steatohepatitis as a novel player in metabolic syndrome-induced erectile dysfunction: an experimental study in the rabbit. Mol Cell Endocrinol 384:143–154
Zhou B, Feng B, Qin Z et al (2016) Activation of farnesoid X receptor downregulates visfatin and attenuates diabetic nephropathy. Mol Cell Endocrinol 419:72–82
Rabinovitch M, Guignabert C, Humbert M, Nicolls MR (2014) Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115:165–175
Guazzi M, Phillips SA, Arena R, Lavie CJ (2015) Endothelial dysfunction and lung capillary injury in cardiovascular diseases. Prog Cardiovasc Dis 57:454–462
Latus H, Delhaas T, Schranz D, Apitz C (2015) Treatment of pulmonary arterial hypertension in children. Nat Rev Cardiol 12:244–254
Lang M, Kojonazarov B, Tian X et al (2012) The soluble guanylate cyclase stimulator riociguat ameliorates pulmonary hypertension induced by hypoxia and SU5416 in rats. PLoS ONE 7:e43433
Malenfant S, Neyron AS, Paulin R et al (2013) Signal transduction in the development of pulmonary arterial hypertension. Pulm Circ 3:278–293
Jing ZC, Parikh K, Pulido T et al (2013) Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 127:624–633
Sitbon O, Channick R, Chin KM et al (2015) Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 373:2522–2533
McLaughlin VV, Benza RL, Rubin LJ et al (2010) Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 55:1915–1922
Ataya A, Cope J, Alnuaimat H (2016) A review of targeted pulmonary arterial hypertension-specific pharmacotherapy. J Clin Med 5(12):E114
Stenmark KR, Meyrick B, Galie N et al (2009) Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297:L1013–L1032
Sakuma F, Miyata M, Kasukawa R (1999) Suppressive effect of prostaglandin E1 on pulmonary hypertension induced by monocrotaline in rats. Lung 177:77–88
Cowan KN, Heilbut A, Humpl T et al (2000) Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 6:698–702
Kwon JH, Kim KC, Cho MS et al (2013) An inhibitory effect of tumor necrosis factor-alpha antagonist to gene expression in monocrotaline-induced pulmonary hypertensive rats model Korean. J Pediatr 56:116–124
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Sasaki Y, Suzuki H, Itoh S et al (2012) K-134, a phosphodiesterase 3 inhibitor, improves gait disturbance and hindlimb blood flow impairment in rat peripheral artery disease models. Eur J Pharmacol 689:132–138
Okumura K, Kato H, Honjo O et al (2015) Carvedilol improves biventricular fibrosis and function in experimental pulmonary hypertension. J Mol Med (Berl) 93:663–674
Comeglio P, Morelli A, Adorini L et al (2017) Beneficial effects of bile acid receptor agonists in pulmonary disease models. Expert Opin Investig Drugs 26:1215–1228
Comeglio P, Filippi S, Sarchielli E et al (2018) Therapeutic effects of obeticholic acid (OCA) treatment in a bleomycin-induced pulmonary fibrosis rat model. J Endocrinol Invest. 2018. https://doi.org/10.1007/s40618-018-0913-1 (epub ahead of print)
Sztuka K, Jasińska-Stroschein M (2017) Animal models of pulmonary arterial hypertension: a systematic review and meta-analysis of data from 6126 animals. Pharmacol Res 125:201–214
Rubin LJ, Badesch DB, Barst RJ et al (2002) Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 346:896–903
Galiè N, Brundage BH, Ghofrani HA et al (2009) Tadalafil therapy for pulmonary arterial hypertension. Circulation 119:2894–2903
Ranchoux B, Antigny F, Rucker-Martin C et al (2015) Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 131:1006–1018
Yang J, Li X, Al-Lamki RS et al (2010) Smad-dependent and smad-independent induction of id1 by prostacyclin analogues inhibits proliferation of pulmonary artery smooth muscle cells in vitro and in vivo. Circ Res 107:252–262
Hashimoto N, Phan SH, Imaizumi K et al (2010) Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 43:161–172
Ahmedat AS, Warnken M, Seemann WK et al (2013) Pro-fibrotic processes in human lung fibroblasts are driven by an autocrine/paracrine endothelinergic system. Br J Pharmacol 168:471–487
Wermuth PJ, Li Z, Mendoza FA, Jimenez SA (2016) Stimulation of transforming growth factor-β1-induced endothelial-to-mesenchymal transition and tissue fibrosis by endothelin-1 (ET-1): a novel profibrotic effect of ET-1. PLoS One 11:e0161988
Breier G, Risau W (1996) The role of vascular endothelial growth factor in blood vessel formation. Trends Cell Biol 6:454–456
Kelland NF, Kuc RE, McLean DL et al (2010) Endothelial cell-specific ETB receptor knockout: autoradiographic and histological characterisation and crucial role in the clearance of endothelin-1. Can J Physiol Pharmacol 88(6):644–651
Leask A (2010) Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res 106(11):1675–1680
Swigris JJ, Brown KK (2010) The role of endothelin-1 in the pathogenesis of idiopathic pulmonary fibrosis. BioDrugs 24(1):49–54
Rosenzweig BL, Imamura T, Okadome T et al (1995) Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA 92:7632–7636
Du L, Sullivan CC, Chu D et al (2003) Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med 348:500–509
Dewachter L, Adnot S, Guignabert C et al (2009) Bone morphogenetic protein signalling in heritable versus idiopathic pulmonary hypertension. Eur Respir J 34:1100–1110
Thomson JR, Machado RD, Pauciulo MW et al (2000) Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 37(10):741–745
Atkinson C, Stewart S, Upton PD et al (2002) Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105:1672–1678
Rouillard AD, Gundersen GW, Fernandez NF et al (2016) The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database (Oxford) 2016:1–16
Shenoy V, Qi Y, Katovich MJ, Raizada MK (2011) ACE2, a promising therapeutic target for pulmonary hypertension. Curr Opin Pharmacol 11(2):150–155
Dai HL, Guo Y, Guang XF et al (2013) The changes of serum angiotensin-converting enzyme 2 in patients with pulmonary arterial hypertension due to congenital heart disease. Cardiology 124:208–212
Ferreira AJ, Shenoy V, Yamazato Y et al (2009) Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med 179(11):1048–1054
Jiang F, Yang J, Zhang Y et al (2014) Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat Rev Cardiol 11:413–426
Yang J, Li X, Al-Lamki RS et al (2013) Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol 33(1):34–42
Thompson AAR, Lawrie A (2017) Targeting vascular remodeling to treat pulmonary arterial hypertension. Trends Mol Med 23(1):31–45
Schermul RT, Kreisselmeier KP, Ghofrani HA et al (2004) Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med 169:39–45
Sawamura F, Kato M, Fujita K et al (2009) Tadalafil, a long-acting inhibitor of PDE5, improves pulmonary hemodynamics and survival rate of monocrotaline-induced pulmonary artery hypertension in rats. J Pharmacol Sci 111:235–243
Lee DS, Kim YK, Jung YW (2010) Simvastatin, sildenafil and their combination in monocrotaline induced pulmonary arterial hypertension. Korean Circ J 40:659–664
Yen CH, Leu S, Lin YC et al (2010) Sildenafil limits monocrotaline-induced pulmonary hypertension in rats through suppression of pulmonary vascular remodeling. J Cardiovasc Pharmacol 55:574–584
Arif SA, Poon H (2011) Tadalafil: a long-acting phosphodiesterase-5 inhibitor for the treatment of pulmonary arterial hypertension. Clin Ther 33:993–1004
Schroll S, Sebah D, Wagner M et al (2013) Improvement of exercise capacity in monocrotaline-induced pulmonary hypertension by the phosphodiesterase-5 inhibitor Vardenafil. Respir Physiol Neurobiol 186:61–64
Acknowledgements
This study has been supported by a scientific Grant from Intercept Pharmaceuticals (New York, NY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PC, SF, ES, AM, IC, CC, GBV, MM and LV have no conflicts of interest. LA is a scientific consultant for Intercept Pharmaceuticals.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Comeglio, P., Filippi, S., Sarchielli, E. et al. Therapeutic effects of the selective farnesoid X receptor agonist obeticholic acid in a monocrotaline-induced pulmonary hypertension rat model. J Endocrinol Invest 42, 951–965 (2019). https://doi.org/10.1007/s40618-019-1009-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-1009-2