Abstract
Purpose
Laparoscopic sleeve gastrectomy (LSG) is one of the most frequently performed bariatric surgery interventions because of its safety and efficacy. Nevertheless, concerns have been raised on its detrimental effect on patient nutritional state that can ultimately lead to the loss of fat-free mass (FFM). There is interest in identifying predictors for the early identification of patients at risk of this highly unwanted adverse because they could benefit of nutritional preventive interventions. Therefore, we investigated whether anthropometric parameters, body composition or resting energy expenditure (REE) measured before surgery could predict FFM loss 1 year after LSG.
Methods
Study design was retrospective observational. We retrieved data on body weight, BMI, body composition and REE before and 1 year after LSG from the medical files of 36 patients operated on by LSG at our institutions. Simple regression, the Oldham’s method and multilevel analysis were used to identify predictors of FFM loss.
Results
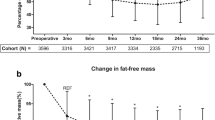
Averaged percentage FFM loss 1 year after LSG was 17.0 ± 7.7% with significant differences between sexes (20.8 ± 6.6 in males and 12.2 ± 6.1% in females, p < 0.001). FFM loss was strongly predicted by pre-surgery FFM and this effect persisted also after correcting for the contribution of sex.
Conclusions
High FFM values before surgery predict a more severe FFM loss after LSG. This factor could also account for the higher FFM loss in men than in women. Our finding could help in the early identification of patient requiring a nutritional support after LSG.






Similar content being viewed by others
References
Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, Toplak H (2015) Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes Facts 8:402–424
De Luca M, Angrisani L, Himpens J, Busetto L, Scopinaro N, Weiner R, Sartori A, Stier C, Lakdawala M, Bhasker AG, Buchwald H, Dixon J, Chiappetta S, Kolberg HC, Frühbeck G, Sarwer DB, Suter M, Soricelli E, Blüher M, Vilallonga R, Sharma A, Shikora S (2016) Indications for surgery for obesity and weight-related diseases: position statements from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg 26:1659–1696
Sjöström L (2013) Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med 273:219–234
Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults. Cochrane Database Syst Rev 8:CD003641
O’Neill KN, Finucane FM, le Roux CW, Fitzgerald AP, Kearney PM (2016) Unmet need for bariatric surgery. Surg Obes Relat Dis 13:1052–1056
Böckelman C, Hahl T, Victorzon M (2017) Mortality following bariatric surgery compared to other common operations in Finland during a 5-year period (2009–2013). A Nationwide Registry Study. Obes Surg 27:2444–2451
Cunningham JJ (1991) Body composition as a determinant of energy expenditure: a synthetic review and a proposed general prediction equation. Am J Clin Nutr 54:963–969
Faria SL, Kelly E, Faria OP (2009) Energy expenditure and weight regain in patients submitted to Roux-en-Y gastric bypass. Obes Surg 19:856–859
Webster JD, Hesp R, Garrow JS (1984) The composition of excess weight in obese women estimated by body density, total body water and total body potassium. Hum Nutr Clin Nutr 38:299–306
Guida B, Belfiore A, Angrisani L, Micanti F, Mauriello C, Trio R, Pecoraro P, Falconi C (2005) Laparoscopic gastric banding and body composition in morbid obesity. Nutr Metab Cardiovasc Dis 15:198–203
Belfiore A, Cataldi M, Minichini L, Aiello ML, Trio R, Rossetti G, Guida B (2015) Short-term changes in body composition and response to micronutrient supplementation after laparoscopic sleeve gastrectomy. Obes Surg 25:2344–2351
Fruhbeck G, Diez Caballero A, Gil MJ (2004) Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med 350:308–309
Sherf Dagan S, Tovim TB, Keidar A, Raziel A, Shibolet O, Zelber-Sagi S (2017) Inadequate protein intake after laparoscopic sleeve gastrectomy surgery is associated with a greater fat free mass loss. Surg Obes Relat Dis 13:101–109
Galloro G, Magno L, Musella M, Manta R, Zullo A, Forestieri P (2014) A novel dedicated endoscopic stent for staple-line leaks after laparoscopic sleeve gastrectomy: a case series. Surg Obes Relat Dis 10:607–611
Musella M, Milone M, Bianco P, Maietta P, Galloro G (2016) Acute leaks following laparoscopic sleeve gastrectomy: early surgical repair according to a management algorithm. J Laparoendosc Adv Surg Tech A 26:85–91
Hayes K, Eid G (2016) Laparoscopic sleeve gastrectomy: surgical technique and perioperative care. Surg Clin North Am 96:763–771
Guida B, Cataldi M, Maresca ID, Germanò R, Trio R, Nastasi AM, Federico S, Memoli A, Apicella L, Memoli B, Sabbatini M (2013) Dietary intake as a link between obesity, systemic inflammation, and the assumption of multiple cardiovascular and antidiabetic drugs in renal transplant recipients. Biomed Res Int 2013:363728
Guida B, De Nicola L, Trio R, Pecoraro P, Iodice C, Memoli B (2000) Comparison of vector and conventional bioelectrical impedance analysis in the optimal dry weight prescription in hemodialysis. Am J Nephrol 20:311–318
Cunningham JJ (1990) Calculation of energy expenditure from indirect calorimetry: assessment of the Weir equation. Nutrition 6:222–223
Compher C, Frankenfield D, Keim N, Roth-Yousey L (2006) Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc 106:881–903
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Leibel RL, Rosenbaum M, Hirsch J (1995) Changes in energy expenditure resulting from altered body weight. N Engl J Med 332:621–628
Ravussin E, Bogardus C (1989) Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr 49(5 Suppl):968–975
Tu YK, Gilthorpe MS (2007) Revisiting the relation between change and initial value: a review and evaluation. Stat Med 26:443–457
Blance A, Tu YK, Gilthorpe MS (2005) A multilevel modelling solution to mathematical coupling. Stat Methods Med Res 14:553–565
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Software 67(1):1–48
Zurlo F, Larson K, Bogardus C, Ravussin E (1990) Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Investig 86:1423–1427
van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT (2010) The muscle fiber type–fiber size paradox: hypertrophy or oxidative metabolism? Eur J Appl Physiol 110:665–694
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91:1447–1531
Wang ZM, Heshka S, Gallagher D, Boozer CN, Kotler DP, Heymsfield SB (2000) Resting energy expenditure–fat-free mass relationship: new insights provided by body composition modeling. Am J Physiol Endocrinol Metab 279:E539–E545
Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S (2013) Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 45:2191–2199
Haizlip KM, Harrison BC, Leinwand LA (2015) Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda) 30:30–39
Lundsgaard AM, Kiens B (2014) Gender differences in skeletal muscle substrate metabolism—molecular mechanisms and insulin sensitivity. Front Endocrinol (Lausanne) 5:195
Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JF, Kiens B (2006) Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol 574:125–138
Hoyenga KB, Hoyenga KT (1982) Gender and energy balance: sex differences in adaptations for feast and famine. Physiol Behav 28:545–563
Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, Dallal GE, Saltzman E (2003) Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr 78:22–30
Carey DG, Pliego GJ, Raymond RL (2006) Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg 16:1602–1608
Maïmoun L, Lefebvre P, Jaussent A, Fouillade C, Mariano-Goulart D, Nocca D (2017) Body composition changes in the first month after sleeve gastrectomy based on gender and anatomic site. Surg Obes Relat Dis 13:780–787
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
For this type of study formal consent is not required.
Informed consent
No informed consent.
Rights and permissions
About this article
Cite this article
Guida, B., Cataldi, M., Busetto, L. et al. Predictors of fat-free mass loss 1 year after laparoscopic sleeve gastrectomy. J Endocrinol Invest 41, 1307–1315 (2018). https://doi.org/10.1007/s40618-018-0868-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0868-2