Abstract
Tumor-induced osteomalacia (TIO), also known as “oncogenic osteomalacia”, is a rare cause of osteomalacia. TIO often has an insidious onset characterized clinically by progressive muscle weakness and bone pain with fractures. The hallmark biochemical finding is a persistent low serum phosphorus concentration due to renal phosphate wasting. The vast majority of cases of TIO result from production of the phosphaturic hormone fibroblast growth factor 23 (FGF23) by a histologically distinctive mesenchymal tumor, termed “phosphaturic mesenchymal tumor” (PMT). Circulating FGF23 induces internalization of renal sodium/phosphate co-transporters resulting in reduced proximal tubular phosphate reabsorption. FGF23 also inhibits production of 1α,25-dihydroxyvitamin D which is inappropriately low or normal in the context of hypophosphatemia. Diagnosis is often delayed owing to the rarity of the condition and an underappreciation for the role of phosphorus as a cause for the constellation of symptoms. Primary treatment for TIO is identification of the offending tumor and surgical removal. However, these tumors are notoriously difficult to find, precluding the opportunity for a curative surgery in many. In such cases, phosphate and calcitriol therapy is used to improve symptoms and heal the osteomalacia. Recently, molecular genetic studies have shown recurrent genetic events in PMT, including the novel fusions FN1–FGFR1 and less commonly FN1–FGF1. These fusion events are hypothesized to result in autocrine/paracrine signaling loops within the tumor, spurring tumorigenesis. This review will cover the clinical features, imaging characteristics, pathologic features, molecular genetic aspects, and therapy of PMT, with a brief discussion of other neoplasms that may cause TIO.

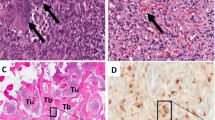
Photographs courtesy of Dr. Yoshinao Oda, Kyushu University, Fukuoka, Japan




Courtesy of Dr. Jen-Chieh Lee, National Taiwan University Hospital, Taipei, Taiwan

Similar content being viewed by others
References
McCance RA (1947) Osteomalacia with Looser’s nodes (Milkman’s syndrome) due to a raised resistance to vitamin D acquired about the age of 15 years. Q J Med 16:33–46
Prader A, Illig R, Uehlinger RE, Stalder G (1959) Rachitis infolge knochentumors (rickets caused by bone tumors). Helv Pediatr Acta 14:554–565
Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY et al (2004) Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol 28(1):1–30
Sundaram M, McCarthy EF (2000) Oncogenic osteomalacia. Skelet Radiol 29(3):117–124
Evans DJ, Azzopardi JG (1972) Distinctive tumours of bone and soft tissue causing acquired vitamin-D-resistant osteomalacia. Lancet 1(7746):353–354
Weidner N, Santa Cruz D (1987) Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer 59(8):1442–1454
Fletcher CDM (2013) WHO classification of tumours of soft tissue and bone. IARC Press, Lyon
Agaimy A, Michal M, Chiosea S, Petersson F, Hadravsky L, Kristiansen G et al (2017) Phosphaturic mesenchymal tumors: clinicopathologic, immunohistochemical and molecular analysis of 22 cases expanding their morphologic and immunophenotypic spectrum. Am J Surg Pathol 41(10):1371–1380
Carter JM, Caron BL, Dogan A, Folpe AL (2015) A novel chromogenic in situ hybridization assay for FGF23 mRNA in phosphaturic mesenchymal tumors. Am J Surg Pathol 39(1):75–83
Zuo QY, Wang H, Li W, Niu XH, Huang YH, Chen J et al (2017) Treatment and outcomes of tumor-induced osteomalacia associated with phosphaturic mesenchymal tumors: retrospective review of 12 patients. BMC Musculoskelet Disord 18(1):403
Hodgson SF, Clarke BL, Tebben PJ, Mullan BP, Cooney WP 3rd, Shives TC (2006) Oncogenic osteomalacia: localization of underlying peripheral mesenchymal tumors with use of Tc 99 m sestamibi scintigraphy. Endocr Pract 12(1):35–42
Wang H, Zhong D, Liu Y, Jiang Y, Qiu G, Weng X et al (2015) Surgical treatments of tumor-induced osteomalacia lesions in long bones: seventeen cases with more than one year of follow-up. J Bone Jt Surg Am 97(13):1084–1094
Hana T, Tanaka S, Nakatomi H, Shojima M, Fukumoto S, Ikemura M et al (2017) Definitive surgical treatment of osteomalacia induced by skull base tumor and determination of the half-life of serum fibroblast growth factor 23. Endocr J 64(10):1033–1039
Kobayashi H, Ito N, Akiyama T, Okuma T, Kinoshita Y, Ikegami M et al (2017) Prevalence and clinical outcomes of hip fractures and subchondral insufficiency fractures of the femoral head in patients with tumour-induced osteomalacia. Int Orthop 41(12):2597–2603
Arai R, Onodera T, Terkawi MA, Mitsuhashi T, Kondo E, Iwasaki N (2017) A rare case of multiple phosphaturic mesenchymal tumors along a tendon sheath inducing osteomalacia. BMC Musculoskelet Disord 18(1):79
Cohen P (1989) The structure and regulation of protein phosphatases. Annu Rev Biochem 58:453–508
Cohen P (1994) The discovery of protein phosphatases: from chaos and confusion to an understanding of their role in cell regulation and human disease. BioEssays 16(8):583–588
Krebs EG, Beavo JA (1979) Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem 48:923–959
Krebs EG, Stull JT (1975) Protein phosphorylation and metabolic control. Ciba Found Symp 31:355–367
Hubbard SR, Till JH (2000) Protein tyrosine kinase structure and function. Annu Rev Biochem 69:373–398
Neuman WF (1980) Bone material and calcification mechanisms. In: Urist MR (ed) Fundamental and clinical bone physiology. Lippincott, Philadelphia, pp 83–107
Berry JL, Davies M, Mee AP (2002) Vitamin D metabolism, rickets, and osteomalacia. Semin Musculoskelet Radiol 6(3):173–182
Econs MJ, McEnery PT (1997) Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab 82(2):674–681
Malloy PJ, Hochberg Z, Tiosano D, Pike JW, Hughes MR, Feldman D (1990) The molecular basis of hereditary 1,25-dihydroxyvitamin D3 resistant rickets in seven related families. J Clin Investig 86(6):2071–2079
Marie PJ, Glorieux FH (1981) Histomorphometric study of bone remodeling in hypophosphatemic vitamin D-resistant rickets. Metab Bone Dis Relat Res 3(1):31–38
Jan de Beur SM (2005) Tumor-induced osteomalacia. JAMA 294(10):1260–1267
Berndt TJ, Knox FG (1992) Renal regulation of phosphate excretion. In: Seldin DW, Giebisch GH (eds) The kidney: physiology and pathophysiology, 2nd edn. Raven Press, New York, pp 2511–2532
Knox FG, Haramati A (1985) Renal regulation of phosphate excretion. In: Seldin DW, Giebisch GH (eds) The kidney: physiology and pathophysiology. Raven Press, New York, pp 1351–1396
Knox FG, Haas JA, Berndt T, Marchand GR, Youngberg SP (1977) Phosphate transport in superficial and deep nephrons in phosphate-loaded rats. Am J Physiol 233(2):F150–F153
Murer H, Hernando N, Forster I, Biber J (2001) Molecular aspects in the regulation of renal inorganic phosphate reabsorption: the type IIa sodium/inorganic phosphate co-transporter as the key player. Curr Opin Nephrol Hypertens 10(5):555–561
Berndt T, Bielesz B, Craig TA, Tebben PJ, Bacic D, Wagner CA et al (2006) Secreted frizzled-related protein-4 reduces sodium-phosphate co-transporter abundance and activity in proximal tubule cells. Pflug Arch Eur J Physiol 451(4):579–587
Taketani Y, Segawa H, Chikamori M, Morita K, Tanaka K, Kido S et al (1998) Regulation of type II renal Na+-dependent inorganic phosphate transporters by 1,25-dihydroxyvitamin D3. Identification of a vitamin D-responsive element in the human NAPi-3 gene. J Biol Chem 273(23):14575–14581
Bhattacharyya N, Chong WH, Gafni RI, Collins MT (2012) Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab 23(12):610–618
Walton RJ, Bijvoet OL (1975) Nomogram for derivation of renal threshold phosphate concentration. Lancet 2(7929):309–310
Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y et al (2003) Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348(17):1656–1663
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T et al (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87(11):4957–4960
Zimering MB, Caldarella FA, White KE, Econs MJ (2005) Persistent tumor-induced osteomalacia confirmed by elevated postoperative levels of serum fibroblast growth factor-23 and 5-year follow-up of bone density changes. Endocr Pract 11(2):108–114
Dupond JL, Mahammedi H, Prie D, Collin F, Gil H, Blagosklonov O et al (2005) Oncogenic osteomalacia: diagnostic importance of fibroblast growth factor 23 and F-18 fluorodeoxyglucose PET/CT scan for the diagnosis and follow-up in one case. Bone 36(3):375–378
Ward LM, Rauch F, White KE, Filler G, Matzinger MA, Letts M et al (2004) Resolution of severe, adolescent-onset hypophosphatemic rickets following resection of an FGF-23-producing tumour of the distal ulna. Bone 34(5):905–911
Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D et al (2006) Sensitivity of fibroblast growth factor 23 measurements in tumor induced osteomalacia. J Clin Endocrinol Metab 91(6):2055–2061
Kobayashi H, Akiyama T, Okuma T, Shinoda Y, Oka H, Ito N et al (2017) Three-dimensional fluoroscopic navigation-assisted surgery for tumors in patients with tumor-induced osteomalacia in the bones. Comput Assist Surg (Abingdon) 22(1):14–19
Bahrami A, Weiss SW, Montgomery E, Horvai AE, Jin L, Inwards CY et al (2009) RT-PCR analysis for FGF23 using paraffin sections in the diagnosis of phosphaturic mesenchymal tumors with and without known tumor induced osteomalacia. Am J Surg Pathol 33(9):1348–1354
Gardner KH, Shon W, Folpe AL, Wieland CN, Tebben PJ, Baum CL (2013) Tumor-induced osteomalacia resulting from primary cutaneous phosphaturic mesenchymal tumor: a case and review of the medical literature. J Cutan Pathol 40(9):780–784 (quiz 79)
Jing H, Li F, Zhuang H, Wang Z, Tian J, Xing X et al (2013) Effective detection of the tumors causing osteomalacia using [Tc-99m]-HYNIC-octreotide (99mTc-HYNIC-TOC) whole body scan. Eur J Radiol 82(11):2028–2034
Jiang Y, Xia WB, Xing XP, Silva BC, Li M, Wang O et al (2012) Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: report of 39 cases and review of the literature. J Bone Miner Res 27(9):1967–1975
Ghorbani-Aghbolaghi A, Darrow MA, Wang T (2017) Phosphaturic mesenchymal tumor (PMT): exceptionally rare disease, yet crucial not to miss. Autops Case Rep 7(3):32–37
Kawai S, Ariyasu H, Furukawa Y, Yamamoto R, Uraki S, Takeshima K et al (2017) Effective localization in tumor-induced osteomalacia using (68)Ga-DOTATOC-PET/CT, venous sampling and 3T-MRI. Endocrinol Diabetes Metab Case Rep 2017. https://doi.org/10.1530/EDM-17-0005
Gonzalez G, Baudrand R, Sepulveda MF, Vucetich N, Guarda FJ, Villanueva P et al (2017) Tumor-induced osteomalacia: experience from a South American academic center. Osteoporos Int 28(7):2187–2193
Singh D, Chopra A, Ravina M, Kongara S, Bhatia E, Kumar N et al (1072) Oncogenic osteomalacia: role of Ga-68 DOTANOC PET/CT scan in identifying the culprit lesion and its management. Br J Radiol 2017(90):20160811
Clifton-Bligh RJ, Hofman MS, Duncan E, Sim Ie W, Darnell D, Clarkson A et al (2013) Improving diagnosis of tumor-induced osteomalacia with Gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab 98(2):687–694
El-Maouche D, Sadowski SM, Papadakis GZ, Guthrie L, Cottle-Delisle C, Merkel R et al (2016) (68)Ga-DOTATATE for tumor localization in tumor-induced osteomalacia. J Clin Endocrinol Metab 101(10):3575–3581
Yamada Y, Kinoshita I, Kenichi K, Yamamoto H, Iwasaki T, Otsuka H et al (2018) Histopathological and genetic review of phosphaturic mesenchymal tumours, mixed connective tissue variant. Histopathology 72(3):460–471
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S et al (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98(11):6500–6505
Jan De Beur SM, Finnegan RB, Vassiliadis J, Cook B, Barberio D, Estes S et al (2002) Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res 17(6):1102–1110
Kumar R (2000) Tumor-induced osteomalacia and the regulation of phosphate homeostasis. Bone 27(3):333–338
Kumar R, Haugen JD, Wieben ED, Londowski JM, Cai Q (1995) Inhibitors of renal epithelial phosphate transport in tumor-induced osteomalacia and uremia. Proc Assoc Am Physicians 107(3):296–305
Cai Q, Hodgson SF, Kao PC, Lennon VA, Klee GG, Zinsmiester AR et al (1994) Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia [comment]. N Engl J Med 330(23):1645–1649
The ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26(3):345–348
Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE et al (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Investig 112(5):683–692
Murer H, Hernando N, Forster I, Biber J (2000) Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 80(4):1373–1409
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K et al (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444(7120):770–774
Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R et al (2001) FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun 284(4):977–981
Perwad F, Zhang MY, Tenenhouse HS, Portale AA (2007) Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Ren Physiol 293(5):F1577–F1583
Sweet RA, Males JL, Hamstra AJ, DeLuca HF (1980) Vitamin D metabolite levels in oncogenic osteomalacia. Ann Intern Med 93(2):279–280
Siris ES, Clemens TL, Dempster DW, Shane E, Segre GV, Lindsay R et al (1987) Tumor-induced osteomalacia. Kinetics of calcium, phosphorus, and vitamin D metabolism and characteristics of bone histomorphometry. Am J Med 82(2):307–312
Leicht E, Biro G, Langer HJ (1990) Tumor-induced osteomalacia: pre- and postoperative biochemical findings. Horm Metab Res 22(12):640–643
Houang M, Clarkson A, Sioson L, Elston MS, Clifton-Bligh RJ, Dray M et al (2013) Phosphaturic mesenchymal tumors show positive staining for somatostatin receptor 2A (SSTR2A). Hum Pathol 44(12):2711–2718
White KE, Larsson TE, Econs MJ (2006) The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev 27(3):221–241
Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R et al (2003) Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Investig 112(5):785–794
Kumar R (2002) New insights into phosphate homeostasis: fibroblast growth factor 23 and frizzled-related protein-4 are phosphaturic factors derived from tumors associated with osteomalacia. Curr Opin Nephrol Hypertens 11(5):547–553
Lee JC, Jeng YM, Su SY, Wu CT, Tsai KS, Lee CH et al (2015) Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J Pathol 235(4):539–545
Lee JC, Su SY, Changou CA, Yang RS, Tsai KS, Collins MT et al (2016) Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod Pathol 29(11):1335–1346
Graham RP, Folpe AL, Oliveira AM, Meyer KJ, Jenkins RB, Sim FH et al (2012) A cytogenetic analysis of two cases of phosphaturic mesenchymal tumor mixed connective tissue type. Hum Pathol 43(8):1334–1338
Parker BC, Engels M, Annala M, Zhang W (2014) Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J Pathol 232(1):4–15
Haviv YS, Silver J (2000) Late onset oncogenic osteomalacia-associated with neurofibromatosis type II. Clin Nephrol 54(5):429–430
Leaf DE, Pereira RC, Bazari H, Juppner H (2013) Oncogenic osteomalacia due to FGF23-expressing colon adenocarcinoma. J Clin Endocrinol Metab 98(3):887–891
Abate EG, Bernet V, Cortese C, Garner HW (2016) Tumor induced osteomalacia secondary to anaplastic thyroid carcinoma: a case report and review of the literature. Bone Rep 5:81–85
Lin HA, Shih SR, Tseng YT, Chen CH, Chiu WY, Hsu CY et al (2014) Ovarian cancer-related hypophosphatemic osteomalacia—a case report. J Clin Endocrinol Metab 99(12):4403–4407
Jin X, Jing H, Li F, Zhuang H (2013) Osteomalacia-inducing renal clear cell carcinoma uncovered by 99mTc-hydrazinonicotinyl-Tyr3-octreotide (99mTc-HYNIC-TOC) scintigraphy. Clin Nucl Med 38(11):922–924
Sauder A, Wiernek S, Dai X, Pereira R, Yudd M, Patel C et al (2016) FGF23-associated tumor-induced osteomalacia in a patient with small cell carcinoma: a case report and regulatory mechanism study. Int J Surg Pathol 24(2):116–120
van Heyningen C, Green AR, MacFarlane IA, Burrow CT (1994) Oncogenic hypophosphataemia and ectopic corticotrophin secretion due to oat cell carcinoma of the trachea. J Clin Pathol 47(1):80–82
Xie Y, Li HZ (2013) Oncogenic osteomalacia caused by renal cell carcinoma. J Clin Endocrinol Metab 98(12):4597–4598
Tella SH, Amalou H, Wood BJ, Chang R, Chen CC, Robinson C et al (2017) Multimodality image-guided cryoablation for inoperable tumor-induced osteomalacia. J Bone Miner Res 32(11):2248–2256
Jan de Beur S, Miller PD, Weber TJ, Peacock M, Ruppe MD, Insogna K, Luca D, Theodore-Oklota C, Martin JS, Carpenter T (2017) Effects of burosumab (KRN23), a human monoclonal antibody to FGF23, in patients with tumor-induced osteomalacia (TIO) or epidermal nevus syndrome (ENS). ASBMR Poster SU0325
Minisola S, Peacock M, Fukumoto S, Cipriani C, Pepe J, Tella SH et al (2017) Tumour-induced osteomalacia. Nat Rev Dis Primers 3:17044
Collins MT (2015) Strking response of tumor-induced osteomalacia to the FGFR inhibitor NVP-BGJ398. In: Annual Meeting of the American Society of Bone and Mineral Research SA0035
Liang G, Chen G, Wei X, Zhao Y, Li X (2013) Small molecule inhibition of fibroblast growth factor receptors in cancer. Cytokine Growth Factor Rev 24(5):467–475
Fukumoto S (2014) Anti-fibroblast growth factor 23 antibody therapy. Curr Opin Nephrol Hypertens 23(4):346–351
Funding
This study received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Boland, Tebben, and Folpe declare that they have no conflicts of interest or relevant financial relationships.
Ethical approval
This manuscript is a review of the literature and does not contain original research either on animal or on human subjects.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Boland, J.M., Tebben, P.J. & Folpe, A.L. Phosphaturic mesenchymal tumors: what an endocrinologist should know. J Endocrinol Invest 41, 1173–1184 (2018). https://doi.org/10.1007/s40618-018-0849-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0849-5