Abstract
Purpose of Review
The phosphaturic hormone FGF23 is produced primarily in osteoblasts/osteocytes and is known to respond to increases in serum phosphate and 1,25(OH)2 vitamin D (1,25D). Novel regulators of FGF23 were recently identified and may help explain the pathophysiologies of several diseases. This review will focus on recent studies examining the synthesis and actions of FGF23.
Recent Findings
The synthesis of FGF23 in response to 1,25D is similar to other steroid hormone targets, but the cellular responses to phosphate remain largely unknown. The activity of intracellular processing genes control FGF23 glycosylation and phosphorylation, providing critical functions in determining the serum levels of bioactive FGF23. The actions of FGF23 largely occur through its co-receptor αKlotho (KL) under normal circumstances, but FGF23 has KL-independent activity during situations of high concentrations.
Summary
Recent work regarding FGF23 synthesis and bioactivity, as well as considerations for diseases of altered phosphate balance, will be reviewed.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as:•• Of major importance
Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–82. https://doi.org/10.1210/endo.143.8.8795.
White KE, Evans WE, O’Riordan JLH, Speer MC, Econs MJ, Lorenz-Depiereux B, et al. ADHR-Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–8.
White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–86. https://doi.org/10.1046/j.1523-1755.2001.00064.x.
•• Bon N, et al. Phosphate-dependent FGF23 secretion is modulated by PiT2/Slc20a2. Mol Metab. 2018;11:197–204. https://doi.org/10.1016/j.molmet.2018.02.007 This reference supports that the phosphate transporter PiT2 is required for producing FGF23 in response to changes in extracellular phosphate.
Perwad F, et al. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–64. https://doi.org/10.1210/en.2005-0777.
Bon N, Couasnay G, Bourgine A, Sourice S, Beck-Cormier S, Guicheux J, et al. Phosphate (Pi)-regulated heterodimerization of the high-affinity sodium-dependent Pi transporters PiT1/Slc20a1 and PiT2/Slc20a2 underlies extracellular Pi sensing independently of Pi uptake. J Biol Chem. 2018;293:2102–14. https://doi.org/10.1074/jbc.M117.807339.
Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, et al. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–42. https://doi.org/10.1152/ajpgi.00243.2005.
Liu S, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–15. https://doi.org/10.1681/ASN.2005111185.
Kaneko I, Saini RK, Griffin KP, Whitfield GK, Haussler MR, Jurutka PW. FGF23 gene regulation by 1,25-dihydroxyvitamin D: opposing effects in adipocytes and osteocytes. J Endocrinol. 2015;226:155–66. https://doi.org/10.1530/JOE-15-0225.
Shimada T, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–35.
Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res. 2014;29:361–9. https://doi.org/10.1002/jbmr.2049.
Zhang Q, Doucet M, Tomlinson RE, Han X, Quarles LD, Collins MT, et al. The hypoxia-inducible factor-1alpha activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 2016;4:16011. https://doi.org/10.1038/boneres.2016.11.
Flamme I, Ellinghaus P, Urrego D, Kruger T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS One. 2017;12:e0186979. https://doi.org/10.1371/journal.pone.0186979.
David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2015;22:1020–32. https://doi.org/10.1038/ki.2015.290.
Ito N, Wijenayaka AR, Prideaux M, Kogawa M, Ormsby RT, Evdokiou A, et al. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol. 2015;399:208–18. https://doi.org/10.1016/j.mce.2014.10.007.
Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108:E1146–55. https://doi.org/10.1073/pnas.1110905108.
Rabadi S, Udo I, Leaf DE, Waikar S, Christov M. Acute blood loss stimulates fibroblast growth factor 23 production. Am J Physiol Ren Physiol. 2017;314(1):F132–9. ajprenal 00081.02017. https://doi.org/10.1152/ajprenal.00081.2017.
Clinkenbeard EL, Hanudel MR, Stayrook KR, Appaiah HN, Farrow EG, Cass TA, et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica. 2017;102:e427–30. https://doi.org/10.3324/haematol.2017.167882.
Hanudel MR, Eisenga MF, Rappaport M, Chua K, Qiao B, Jung G, et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol Dial Transplant. 2018. https://doi.org/10.1093/ndt/gfy189.
Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–803. https://doi.org/10.1002/jbmr.1923.
Shimizu Y, Tada Y, Yamauchi M, Okamoto T, Suzuki H, Ito N, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009;45:814–6. https://doi.org/10.1016/j.bone.2009.06.017.
Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94:2332–7. https://doi.org/10.1210/jc.2008-2396.
Smith RC, O’Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, et al. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. 2012;122:4710–5. https://doi.org/10.1172/JCI64986.
Hum JM, et al. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble Klotho. J Am Soc Nephrol. 2016;28:1162–74. https://doi.org/10.1681/ASN.2015111266.
Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–31. https://doi.org/10.1677/JOE-07-0267.
Knab VM, Corbin B, Andrukhova O, Hum JM, Ni P, Rabadi S, et al. Acute parathyroid hormone injection increases C-terminal but not intact fibroblast growth factor 23 levels. Endocrinology. 2017;158:1130–9. https://doi.org/10.1210/en.2016-1451.
Kobayashi K, et al. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol. 2006;154:93–9. https://doi.org/10.1530/eje.1.02053.
Ben-Dov IZ, et al. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–8. https://doi.org/10.1172/JCI32409.
David V, et al. Calcium regulates FGF-23 expression in bone. Endocrinology. 2013;154:4469–82. https://doi.org/10.1210/en.2013-1627.
Clinkenbeard EL, Cass TA, Ni P, Hum JM, Bellido T, Allen MR, et al. Conditional deletion of murine Fgf23: interruption of the normal skeletal responses to phosphate challenge and rescue of genetic hypophosphatemia. J Bone Miner Res. 2016;31:1247–57. https://doi.org/10.1002/jbmr.2792.
•• Onal M, et al. A novel distal enhancer mediates inflammation-, PTH-, and early onset murine kidney disease-induced expression of the mouse Fgf23 gene. JBMR Plus. 2018;2:32–47. https://doi.org/10.1002/jbm4.10023 This citation demonstrates that distal portions of the FGF23 promoter may regulate FGF23 under specific physiological and disease conditions.
Shimada T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8. https://doi.org/10.1172/JCI19081.
Fleet JC, Replogle RA, Reyes-Fernandez P, Wang L, Zhang M, Clinkenbeard EL, et al. Gene-by-diet interactions affect serum 1,25-dihydroxyvitamin D levels in male BXD recombinant inbred mice. Endocrinology. 2016;157:470–81. https://doi.org/10.1210/en.2015-1786.
Gravesen E, Mace ML, Hofman-Bang J, Olgaard K, Lewin E. Circulating FGF23 levels in response to acute changes in plasma Ca(2+). Calcif Tissue Int. 2014;95:46–53. https://doi.org/10.1007/s00223-014-9861-8.
Rodriguez-Ortiz ME, et al. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 2012;23:1190–7. https://doi.org/10.1681/ASN.2011101006.
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4. https://doi.org/10.1038/nature05315.
Kawata T, et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–8. https://doi.org/10.1681/ASN.2006070783.
Meir T, Durlacher K, Pan Z, Amir G, Richards WG, Silver J, et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 2014;86:1106–15. https://doi.org/10.1038/ki.2014.215.
Hanks LJ, Casazza K, Judd SE, Jenny NS, Gutierrez OM. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS One. 2015;10:e0122885. https://doi.org/10.1371/journal.pone.0122885.
Holecki M, et al. Inflammation but not obesity or insulin resistance is associated with increased plasma fibroblast growth factor 23 concentration in the elderly. Clin Endocrinol. 2015;82:900–9. https://doi.org/10.1111/cen.12759.
Eren M, Place AT, Thomas PM, Flevaris P, Miyata T, Vaughan DE. PAI-1 is a critical regulator of FGF23 homeostasis. Sci Adv. 2017;3:e1603259. https://doi.org/10.1126/sciadv.1603259.
Nam KH, Kim H, An SY, Lee M, Cha MU, Park JT, et al. Circulating fibroblast growth factor-23 levels are associated with an increased risk of anemia development in patients with nondialysis chronic kidney disease. Sci Rep. 2018;8:7294. https://doi.org/10.1038/s41598-018-25439-z.
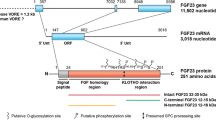
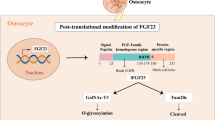
Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A. 2014;111:5520–5. https://doi.org/10.1073/pnas.1402218111.
Kato K, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281:18370–7. https://doi.org/10.1074/jbc.M602469200.
Ichikawa S, et al. Genetic rescue of glycosylation-deficient Fgf23 in the Galnt3 knockout mouse. Endocrinology. 2014;155:3891–8. https://doi.org/10.1210/en.2014-1199.
Raine J, Winter RM, Davey A, Tucker SM. Unknown syndrome: microcephaly, hypoplastic nose, exophthalmos, gum hyperplasia, cleft palate, low set ears, and osteosclerosis. J Med Genet. 1989;26:786–8.
Simpson MA, Hsu R, Keir LS, Hao J, Sivapalan G, Ernst LM, et al. Mutations in FAM20C are associated with lethal osteosclerotic bone dysplasia (Raine syndrome), highlighting a crucial molecule in bone development. Am J Hum Genet. 2007;81:906–12. https://doi.org/10.1086/522240.
Wang X, Wang S, Li C, Gao T, Liu Y, Rangiani A, et al. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLoS Genet. 2012;8:e1002708. https://doi.org/10.1371/journal.pgen.1002708.
Carpenter TO, et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J Clin Invest. 2014;124:1587–97. https://doi.org/10.1172/JCI72829.
Imel EA, et al. Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J Clin Endocrinol Metab. 2015;100:2565–73. https://doi.org/10.1210/jc.2015-1551.
Carpenter TO, Whyte MP, Imel EA, Boot AM, Högler W, Linglart A, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378:1987–98. https://doi.org/10.1056/NEJMoa1714641.
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima YI. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242:626–30.
•• Chen G, et al. alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553:461–6. https://doi.org/10.1038/nature25451 This paper reported the triple crystal structure of FGF23-KL-FGFR1, and tests the idea that the soluble form of KL can mediate FGF23 bioactivity in tissue where KL has limited expression.
Imura A, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–7. https://doi.org/10.1016/j.febslet.2004.03.090S0014579304003990.
Hu MC, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–36. https://doi.org/10.1681/ASN.2009121311.
Gattineni J, Alphonse P, Zhang Q, Mathews N, Bates CM, Baum M. Regulation of renal phosphate transport by FGF23 is mediated by FGFR1 and FGFR4. Am J Physiol Ren Physiol. 2014;306:F351–8. https://doi.org/10.1152/ajprenal.00232.2013.
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51.
Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009;20:955–60. https://doi.org/10.1681/ASN.2008070783.
Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren Ö, Tenenhouse HS, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–94.
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98:6500–5.
Olauson H, et al. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol. 2012;23:1641–51. https://doi.org/10.1681/ASN.2012010048.
Ide N, Olauson H, Sato T, Densmore MJ, Wang H, Hanai JI, et al. In vivo evidence for a limited role of proximal tubular Klotho in renal phosphate handling. Kidney Int. 2016;90:348–62. https://doi.org/10.1016/j.kint.2016.04.009.
Takeshita A, Kawakami K, Furushima K, Miyajima M, Sakaguchi K. Central role of the proximal tubular alphaKlotho/FGF receptor complex in FGF23-regulated phosphate and vitamin D metabolism. Sci Rep. 2018;8:6917. https://doi.org/10.1038/s41598-018-25087-3.
Ide N, Ye R, Courbebaisse M, Olauson H, Densmore MJ, Larsson TE, et al. In vivo evidence for an interplay of FGF23/Klotho/PTH axis on the phosphate handling in renal proximal tubules. Am J Physiol Ren Physiol. 2018;315:F1261–70. https://doi.org/10.1152/ajprenal.00650.2017.
Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6:744–59. https://doi.org/10.1002/emmm.201303716.
Chang Q, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–3.
Andrukhova O, et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 2013;33:229–46. https://doi.org/10.1002/embj.201284188.
Olauson H, Mencke R, Hillebrands JL, Larsson TE. Tissue expression and source of circulating alphaKlotho. Bone. 2017;100:19–35. https://doi.org/10.1016/j.bone.2017.03.043.
Faul C, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–408. https://doi.org/10.1172/JCI46122.
Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, et al. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22:1020–32. https://doi.org/10.1016/j.cmet.2015.09.002.
•• Grabner A, et al. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci Rep. 2017;7:1993. https://doi.org/10.1038/s41598-017-02068-6 This paper showed that the cardiac hypertrophy due to high-phopshate diet and elevated FGF23 could be reversed by lowering serum phopshate.
Czaya B, et al. Induction of an inflammatory response in primary hepatocyte cultures from mice. J Visual Exp. 2017. https://doi.org/10.3791/55319.
Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90:985–96. https://doi.org/10.1016/j.kint.2016.05.019.
Liu ES, Thoonen R, Petit E, Yu B, Buys ES, Scherrer-Crosbie M, et al. Increased circulating FGF23 does not lead to cardiac hypertrophy in the male Hyp mouse model of XLH. Endocrinology. 2018;159:2165–72. https://doi.org/10.1210/en.2018-00174.
Neuburg S, Dussold C, Gerber C, Wang X, Francis C, Qi L, et al. Genetic background influences cardiac phenotype in murine chronic kidney disease. Nephrol Dial Transplant. 2018;33:1129–37. https://doi.org/10.1093/ndt/gfx332.
Acknowledgements
KEW receives royalties for licensing the FGF23 gene to Kyowa Hakko Kirin, Ltd.; the authors would like to acknowledge support by NIH grants DK112958, DK095784, and AR059278 (KEW), and T32-HL007910 (MLN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Megan L. Noonan and Kenneth E. White each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Molecular Control of Phosphorus Homeostasis
Rights and permissions
About this article
Cite this article
Noonan, M.L., White, K.E. FGF23 Synthesis and Activity. Curr Mol Bio Rep 5, 18–25 (2019). https://doi.org/10.1007/s40610-019-0111-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-019-0111-8