Abstract
Purpose of Review
Fibroblast growth factor-23 (FGF23) is the key hormone produced in bone critical for phosphate homeostasis. Elevated serum phosphorus and 1,25-dihydroxyvitamin D stimulates FGF23 production to promote renal phosphate excretion and decrease 1,25-dihydroxyvitamin D synthesis, thus completing the feedback loop and suppressing FGF23. Unexpectedly, studies of common and rare heritable disorders of phosphate handling identified links between iron and FGF23 demonstrating novel regulation outside the phosphate pathway.
Recent Findings
Iron deficiency combined with an FGF23 cleavage mutation was found to induce the autosomal dominant hypophosphatemic rickets phenotype. Physiological responses to iron deficiency, such as erythropoietin production as well as hypoxia inducible factor activation, have been indicated in regulating FGF23. Additionally, specific iron formulations, used to treat iron deficiency, alter post-translational processing thereby shifting FGF23 protein secretion.
Summary
Molecular and clinical studies revealed that iron deficiency, through several mechanisms, alters FGF23 at the transcriptional and post-translational level. This review will focus upon the novel discoveries elucidated between iron, its regulators, and their influence on FGF23 bioactivity.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98(11):6500–5.
Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18(7):1227–34.
Yu X, White KE. FGF23 and disorders of phosphate homeostasis. Cytokine Growth Factor Rev. 2005;16(2):221–32.
Cho HY, Lee BH, Kang JH, Ha IS, Cheong HI, Choi Y. A clinical and molecular genetic study of hypophosphatemic rickets in children. Pediatr Res. 2005;58(2):329–33.
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–7.
Yu X, White KE. Fibroblast growth factor 23 and its receptors. Ther Apher Dial. 2005;9(4):308–12.
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4.
Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15(4):437–41.
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–3.
Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, et al. Alpha-klotho is a non- enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553(7689):461–6.
Farrow EG, Summers LJ, Schiavi SC, McCormick JA, Ellison DH, White KE. Altered renal FGF23-mediated activity involving MAPK and Wnt: effects of the Hyp mutation. J Endocrinol. 2010;207(1):67–75.
Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008;23(9):1509–18.
Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, et al. Human fibroblast growth factor-23 mutants suppress Na+−dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278(4):2206–11.
Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006;69(3):495–503.
Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17(5):1305–15.
Econs MJ, McEnery PT, Lennon F, Speer MC. Autosomal dominant hypophosphatemic rickets is linked to chromosome 12p13. J Clin Invest. 1997;100(11):2653–7.
ADHR-Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 2000;26(3):345–348.
Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36(6):579–81.
Garringer HJ, Fisher C, Larsson TE, Davis SI, Koller DL, Cullen MJ, et al. The role of mutant UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab. 2006;91(10):4037–42.
Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, et al. Hyperostosis- hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22(2):235–42.
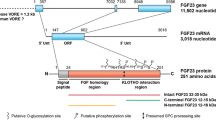
Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A. 2014;111(15):5520–5.
Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(1):396–9.
Lefebvre P, Vekeman F, Sarokhan B, Enny C, Provenzano R, Cremieux PY. Relationship between hemoglobin level and quality of life in anemic patients with chronic kidney disease receiving epoetin alfa. Curr Med Res Opin. 2006;22(10):1929–37.
Jamal SA. Bone mass measurements in men and women with chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19(4):343–8.
Rolvien T, Kornak U, Schinke T, Amling M, Oheim R. A novel FAM20C mutation causing hypophosphatemic osteomalacia with osteosclerosis (mild Raine syndrome) in an elderly man with spontaneous osteonecrosis of the knee. Osteoporos Int. 2018.
Min JH, Yang H, Ivan M, Gertler F, Kaelin WG Jr, Pavletich NP. Structure of an HIF- 1alpha -pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296(5574):1886–9.
Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the third National Health and nutrition examination survey. J Am Soc Nephrol. 2002;13(2):504–10.
Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–5.
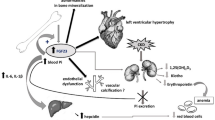
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408.
Grabner A, Mazzaferro S, Cianciolo G, Krick S, Capelli I, Rotondi S, et al. Fibroblast growth factor 23: mineral metabolism and beyond. Contrib Nephrol. 2017;190:83–95.
Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108(46):E1146–55.
Van Buren PN, Lewis JB, Dwyer JP, Greene T, Middleton J, Sika M, et al. The phosphate binder ferric citrate and mineral metabolism and inflammatory markers in maintenance Dialysis patients: results from Prespecified analyses of a randomized clinical trial. Am J Kidney Dis. 66(3):479–88.
Geissler C, Singh M. Iron, meat and health. Nutrients. 2011;3(3):283–316.
McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, et al. An iron- regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291(5509):1755–9.
Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85.
Brannon PM, Taylor CL. Iron Supplementation during Pregnancy and Infancy: uncertainties and implications for research and policy. Nutrients. 2017;9(12).
Muller O, Krawinkel M. Malnutrition and health in developing countries. CMAJ. 2005;173(3):279–86.
Skalicky A, Meyers AF, Adams WG, Yang Z, Cook JT, Frank DA. Child food insecurity and iron deficiency anemia in low-income infants and toddlers in the United States. Matern Child Health J. 2006;10(2):177–85.
Diaz-Castro J, Lopez-Frias MR, Campos MS, Lopez-Frias M, Alferez MJ, Nestares T, et al. Severe nutritional iron-deficiency anaemia has a negative effect on some bone turnover biomarkers in rats. Eur J Nutr. 2012;51(2):241–7.
Cartwright GE, Lauritsen MA, Humphreys S, Jones PJ, Merrill IM, Wintrobe MM. The Anemia associated with chronic infection. Science. 1946;103(2664):72–3.
Cartwright GE, Lauritsen MA, Jones PJ, Merrill IM, Wintrobe MM. The Anemia of infection. I. Hypoferremia, hypercupremia, and alterations in porphyrin metabolism in patients. J Clin Invest. 1946;25(1):65–80.
Qamar K, Saboor M, Qudsia F, Khosa SM. Moinuddin, Usman M. Malabsorption of iron as a cause of iron deficiency anemia in postmenopausal women. Pak J Med Sci. 2015;31(2):304–8.
Filmann N, Rey J, Schneeweiss S, Ardizzone S, Bager P, Bergamaschi G, et al. Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20(5):936–45.
Gotloib L, Silverberg D, Fudin R, Shostak A. Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J Nephrol. 2006;19(2):161–7.
Lankhorst CE, Wish JB. Anemia in renal disease: diagnosis and management. Blood Rev. 2010;24(1):39–47.
Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82(2):674–81.
Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22(4):520–6.
Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant Hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96:3541–9.
Vieth JT, Lane DR. Anemia. Emerg Med Clin North Am. 2014;32(3):613–28.
Bryan LJ, Zakai NA. Why is my patient anemic? Hematol Oncol Clin North Am. 2012;26(2):205–30 vii.
Rabadi S, Udo I, Leaf DE, Waikar SS, Christov M. Acute blood loss stimulates fibroblast growth factor 23 production. Am J Physiol Renal Physiol. 2018;314(1):F132–F9.
Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793–803.
Lewerin C, Ljunggren O, Nilsson-Ehle H, Karlsson MK, Herlitz H, Lorentzon M, et al. Low serum iron is associated with high serum intact FGF23 in elderly men: the Swedish MrOS study. Bone. 2017;98:1–8.
Erlitzki R, Long JC, Theil EC. Multiple, conserved iron-responsive elements in the 3′- untranslated region of transferrin receptor mRNA enhance binding of iron regulatory protein 2. J Biol Chem. 2002;277(45):42579–87.
Thomson AM, Rogers JT, Leedman PJ. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. Int J Biochem Cell Biol. 1999;31(10):1139–52.
Piccinelli P, Samuelsson T. Evolution of the iron-responsive element. RNA. 2007;13(7):952–66.
Sanchez M, Galy B, Schwanhaeusser B, Blake J, Bahr-Ivacevic T, Benes V, et al. Iron regulatory protein-1 and -2: transcriptome-wide definition of binding mRNAs and shaping of the cellular proteome by iron regulatory proteins. Blood. 2011;118(22):e168–79.
Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90(9):4304–8.
Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117(7):1926–32.
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72.
Ivan M, Haberberger T, Gervasi DC, Michelson KS, Gunzler V, Kondo K, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A. 2002;99(21):13459–64.
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL- mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8.
Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High- resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117(23):e207–17.
Bailey PSJ, Nathan JA. Metabolic Regulation of Hypoxia-Inducible Transcription Factors: The Role of Small Molecule Metabolites and Iron. Biomedicines. 2018;6(2).
Bianchi L, Tacchini L, Cairo G. HIF-1-mediated activation of transferrin receptor gene transcription by iron chelation. Nucleic Acids Res. 1999;27(21):4223–7.
Weinberg ED. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984;64(1):65–102.
David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135–46.
Zhang Q, Doucet M, Tomlinson RE, Han X, Quarles LD, Collins MT, et al. The hypoxia- inducible factor-1alpha activates ectopic production of fibroblast growth factor 23 in tumor- induced osteomalacia. Bone Res. 2016;4:16011.
•• Onal M, Carlson AH, Thostenson JD, Benkusky NA, Meyer MB, Lee SM, et al. A Novel Distal Enhancer Mediates Inflammation-, PTH-, and Early Onset Murine Kidney Disease- Induced Expression of the Mouse Fgf23 Gene. JBMR Plus. 2018;2(1):32–47 This study demonstrated the regulation of FGF23 by a distal upstream enhancer.
Bruning U, Fitzpatrick SF, Frank T, Birtwistle M, Taylor CT, Cheong A. NFkappaB and HIF display synergistic behaviour during hypoxic inflammation. Cell Mol Life Sci. 69(8):1319–29.
Jacobson LO, Goldwasser E, Fried W, Plzak L. Role of the kidney in erythropoiesis. Nature. 1957;179(4560):633–4.
Franke K, Gassmann M, Wielockx B. Erythrocytosis: the HIF pathway in control. Blood. 2013;122(7):1122–8.
Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem. 2000;275(38):29338–47.
Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–84.
Arezes J, Foy N, McHugh K, Sawant A, Quinkert D, Terraube V, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132:1473–7.
Daryadel A, Bettoni C, Haider T, Imenez Silva PH, Schnitzbauer U, Pastor-Arroyo EM, et al. Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflugers Arch. 2018;470:1569–82.
Hanudel MR, Eisenga MF, Rappaport M, Chua K, Qiao B, Jung G, et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol Dial Transplant. 2018.
Gupta N, Wish JB. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for Anemia in patients with CKD. Am J Kidney Dis. 2017;69(6):815–26.
Flamme I, Ellinghaus P, Urrego D, Kruger T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS One. 2017;12(10):e0186979.
Toro L, Barrientos V, Leon P, Rojas M, Gonzalez M, Gonzalez-Ibanez A, et al. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int. 2018;93(5):1131–41.
Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23(10):1631–4.
Hinata A, Iijima M, Nakano Y, Sakamoto T, Tomita M. Chemical characterization of rabbit alpha 2-macroglobulin. Chem Pharm Bull (Tokyo). 1987;35(1):271–6.
Landau D, London L, Bandach I, Segev Y. The hypoxia inducible factor/erythropoietin (EPO)/EPO receptor pathway is disturbed in a rat model of chronic kidney disease related anemia. PLoS One. 2018;13(5):e0196684.
•• Clinkenbeard EL, Hanudel MR, Stayrook KR, Appaiah HN, Farrow EG, Cass TA, et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica. 2017;102(11):e427–e30 This study demonstrated EPO stimulation of FGF23 independent of HIF that occurs in both osteoblast/osteocytes as well as hematopoietic lineage cells.
Singbrant S, Russell MR, Jovic T, Liddicoat B, Izon DJ, Purton LE, et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 2011;117(21):5631–42.
Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res. 2014;29(2):361–9.
Duval F, Mokrani MC, Monreal J, Weiss T, Fattah S, Hamel B, et al. Interaction between the serotonergic system and HPA and HPT axes in patients with major depression: implications for pathogenesis of suicidal behavior. Dialogues Clin Neurosci. 2002;4(4):417.
Okada M, Imamura K, Fuchigami T, Omae T, Iida M, Nanishi F, et al. 2 cases of nonspecific multiple ulcers of the small intestine associated with osteomalacia caused by long- term intravenous administration of saccharated ferric oxide. Nihon Naika Gakkai zasshi The Journal of the Japanese Society of Internal Medicine. 1982;71(11):1566–72.
Auerbach M, Macdougall IC. Oral Iron therapy: after three centuries, it is time for a change. Am J Kidney Dis. 2016;68(5):665–6.
Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3(1):12–33.
Bishay RH, Ganda K, Seibel MJ. Long-term iron polymaltose infusions associated with hypophosphataemic osteomalacia: a report of two cases and review of the literature. Ther Adv Endocrinol Metab. 2017;8(1–2):14–9.
Gilmartin CE, Hoang T, Cutts BA, Leung L. Retrospective cohort study comparing the adverse reactions and efficacy of intravenous iron polymaltose with ferric carboxymaltose for iron deficiency anemia. Int J Gynaecol Obstet. 2018;141(3):315–20.
Urbina T, Belkhir R, Rossi G, Carbonnel F, Pavy S, Collins M, et al. Iron supplementation-induced Phosphaturic Osteomalacia: FGF23 is the culprit. J Bone Miner Res. 2018;33(3):540–2.
Bartko J, Roschger P, Zandieh S, Brehm A, Zwerina J, Klaushofer K. Hypophosphatemia, severe bone pain, gait disturbance, and fatigue fractures after Iron substitution in inflammatory bowel disease: a case report. J Bone Miner Res. 2018;33(3):534–9.
Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018;2018.
Tulewicz-Marti E, Moniuszko A, Rydzewska G. Management of anemia in inflammatory bowel disease: a challenge in everyday clinical practice. Przeglad gastroenterologiczny. 2017;12(4):239–43.
Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92.
Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–9.
Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol. 2011;22(5):956–66.
Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207(2):546–51.
Fukao W, Hasuike Y, Yamakawa T, Toyoda K, Aichi M, Masachika S, et al. Oral versus intravenous Iron supplementation for the treatment of Iron deficiency Anemia in patients on maintenance hemodialysis-effect on fibroblast growth Factor-23 metabolism. J Renal Nutr. 2018;28(4):270–7.
Liabeuf S, Ryckelynck JP, El Esper N, Urena P, Combe C, Dussol B, et al. Randomized clinical trial of Sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin J Am Soc Nephrol. 2017;12(12):1930–40.
Koiwa F, Yokoyama K, Fukagawa M, Terao A, Akizawa T. Efficacy and safety of sucroferric oxyhydroxide compared with sevelamer hydrochloride in Japanese haemodialysis patients with hyperphosphataemia: a randomized, open-label, multicentre, 12-week phase III study. Nephrology (Carlton). 2017;22(4):293–300.
Covic AC, Floege J, Ketteler M, Sprague SM, Lisk L, Rakov V, et al. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transplant. 2017;32(8):1330–8.
Yang WC, Yang CS, Hou CC, Wu TH, Young EW, Hsu CH. An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: ferric citrate. Nephrol Dial Transplant. 2002;17(2):265–70.
Lee CT, Wu IW, Chiang SS, Peng YS, Shu KH, Wu MJ, et al. Effect of oral ferric citrate on serum phosphorus in hemodialysis patients: multicenter, randomized, double-blind, placebo- controlled study. J Nephrol. 2015;28(1):105–13.
Maruyama N, Otsuki T, Yoshida Y, Nagura C, Kitai M, Shibahara N, et al. Ferric citrate decreases fibroblast growth factor 23 and improves erythropoietin responsiveness in hemodialysis patients. Am J Nephrol. 2018;47(6):406–14.
Iguchi A, Yamamoto S, Yamazaki M, Tasaki K, Suzuki Y, Kazama JJ, et al. Effect of ferric citrate hydrate on FGF23 and PTH levels in patients with non-dialysis-dependent chronic kidney disease with normophosphatemia and iron deficiency. Clin Exp Nephrol. 2018;22(4):789–96.
Funding
The authors would like to acknowledge NIH grants F32-AR065389 (ELC); the Comprehensive Training Program in Musculoskeletal Research Grant T32-AR065971 (JAW) and a Center for Translational Sciences Institutional Biomedical Research Grant (ELC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jonathan A. Wheeler and Erica L. Clinkenbeard each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Molecular Control of Phosphorus Homeostasis
Rights and permissions
About this article
Cite this article
Wheeler, J.A., Clinkenbeard, E.L. Regulation of Fibroblast Growth Factor 23 by Iron, EPO, and HIF. Curr Mol Bio Rep 5, 8–17 (2019). https://doi.org/10.1007/s40610-019-0110-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-019-0110-9