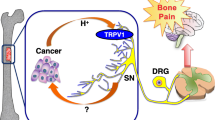
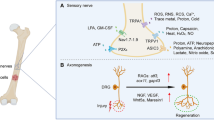
Abstract
Purpose of Review
Majority of patients with solid and hematologic cancers associated with osteolytic bone disease suffer from severe uncontrollable bone pain. Treatment of bone pain is an important goal in the management of these cancer patients. However, our understanding of the mechanism underlying cancer-associated bone pain (CABP) is limited and current treatments for CABP are ineffective and unsatisfactory. In this review, the pathophysiology of CABP will be discussed with a special focus on cancer-created acidic bone microenvironment as a potential therapeutic target.
Recent Findings
Recent accumulating findings that sensory nerves (SNs) densely innervate bone suggest that CABP can be induced as a consequence of SN activation by the pathologic changes in bone microenvironment. Cancer cells proliferating in bone secrete protons and lactate resulting from the Warburg effect, creating acidic bone microenvironment. In parallel, cancer in bone increases and activates osteoclasts, which release protons to degrade bone minerals, also making bone microenvironment acidic. The acidic bone microenvironment sensitizes and excites bone-innervating SNs to evoke CABP via upregulation and activation of the acid-sensing nociceptors such as ASIC3 and TRPV1 expressed on SNs. Blockade of the creation of the acidic bone microenvironment and/or interruption of the activation of these nociceptors decrease SN stimulation and CABP.
Summary
Determination of the mechanism by which the acidic cancer microenvironment is generated and by which SN is excited and sensitized via activation of the acid-sensing nociceptors would promote to design mechanism-based novel and effective therapeutic interventions for the management of CABP.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–9s. https://doi.org/10.1158/1078-0432.ccr-06-0931.
Rizzoli R, Body JJ, Brandi ML, Cannata-Andia J, Chappard D, El Maghraoui A, et al. Cancer-associated bone disease. Osteoporos Int. 2013;24(12):2929–53. https://doi.org/10.1007/s00198-013-2530-3.
Burrows M, Dibble SL, Miaskowski C. Differences in outcomes among patients experiencing different types of cancer-related pain. Oncol Nurs Forum. 1998;25(4):735–41.
Poulos AR, Gertz MA, Pankratz VS, Post-White J. Pain, mood disturbance, and quality of life in patients with multiple myeloma. Oncol Nurs Forum. 2001;28(7):1163–71.
•• Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69(1–2):1–18. This paper is one of the earliest articles that introduced the clinical and basic features of CABP
Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, et al. National Institutes of Health State-of-the-Science Conference statement: symptom management in cancer: pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst. 2003;95(15):1110–7.
•• Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7(10):797–809. https://doi.org/10.1038/nrn1914. This paper dissected and characterized CABP based on preclinical studies using animal models of bone cancer
Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol. 2014;32(16):1647–54. https://doi.org/10.1200/jco.2013.51.7219.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–84. https://doi.org/10.1016/j.cell.2009.09.028.
Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–66.
•• Hiasa M, Okui T, Allette YM, Ripsch MS, Sun-Wada GH, Wakabayashi H, et al. Bone pain induced by multiple myeloma is reduced by targeting V-ATPase and ASIC3. Cancer Res. 2017;77(6):1283–95. https://doi.org/10.1158/0008-5472.can-15-3545. This study shows the importance of the creation of acidic cancer environment and the acid-sensing nociceptor ASIC3 in CABP associated with multiple myeloma
• Wakabayashi H, Wakisaka S, Hiraga T, Hata K, Nishimura R, Tominaga M, Yoneda T Decreased sensory nerve excitation and bone pain associated with mouse Lewis lung cancer in TRPV1-deficient mice. J Bone Miner Metab 2017. https://doi.org/10.1007/s00774-017-0842-7. This work demonstrates critical role of the acid-sensing nociceptor TRPV1 in CABP associated lung cancer using TRPV1 −/− mice.
Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol. 2009;9(1):9–14. https://doi.org/10.1016/j.coph.2008.12.007.
Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat Neurosci. 2001;4(4):357–8. https://doi.org/10.1038/86001.
Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171(2):588–98. https://doi.org/10.1016/j.neuroscience.2010.08.056.
Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci. 2010;30(44):14649–56. https://doi.org/10.1523/jneurosci.3300-10.2010.
Johnson RW, Suva LJ. Hallmarks of bone metastasis. Calcif Tissue Int. 2017;102:141–51. https://doi.org/10.1007/s00223-017-0362-4.
Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab. 2007;25(2):99–104. https://doi.org/10.1007/s00774-006-0734-8.
Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–10. https://doi.org/10.1038/35093019.
Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci. 2014;39(3):508–19. https://doi.org/10.1111/ejn.12462.
Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 2015;18(1):24–32; discussion 32. https://doi.org/10.1111/ner.12247.
Cooper RR. Nerves in cortical bone. Science. 1968;160(3825):327–8.
Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25(6):623–9.
Irie K, Hara-Irie F, Ozawa H, Yajima T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Tech. 2002;58(2):85–90. https://doi.org/10.1002/jemt.10122.
Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497(7450):490–3. https://doi.org/10.1038/nature12115.
Paolucci T, Saraceni VM, Piccinini G. Management of chronic pain in osteoporosis: challenges and solutions. J Pain Res. 2016;9:177–86. https://doi.org/10.2147/jpr.s83574.
Muralidharan A, Smith MT. Pathobiology and management of prostate cancer-induced bone pain: recent insights and future treatments. Inflammopharmacology. 2013;21(5):339–63. https://doi.org/10.1007/s10787-013-0183-7.
Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW. Cancer-induced bone pain: mechanisms and models. Neurosci Lett. 2013;557(Pt A):52–9. https://doi.org/10.1016/j.neulet.2013.08.003.
Yoneda T, Hiasa M, Nagata Y, Okui T, White FA. Contribution of acidic extracellular microenvironment of cancer-colonized bone to bone pain. Biochim Biophys Acta. 2015;1848(10 Pt B):2677–84. https://doi.org/10.1016/j.bbamem.2015.02.004.
Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol. 2012;8(6):358–66. https://doi.org/10.1038/nrrheum.2012.36.
Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9(4):285–96. https://doi.org/10.1038/nrm2354.
Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–77. https://doi.org/10.1038/nrd3554.
Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13(9):611–23. https://doi.org/10.1038/nrc3579.
Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–8.
Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–93. https://doi.org/10.1038/nrc867.
Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–64. https://doi.org/10.1056/NEJMra030831.
Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–25. https://doi.org/10.1038/nrc3055.
Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328(3):679–87. https://doi.org/10.1016/j.bbrc.2004.11.070.
Cleeland CS, Body JJ, Stopeck A, von Moos R, Fallowfield L, Mathias SD, et al. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119(4):832–8. https://doi.org/10.1002/cncr.27789.
• von Moos R, Costa L, Ripamonti CI, Niepel D, Santini D. Improving quality of life in patients with advanced cancer: targeting metastatic bone pain. Eur J Cancer. 2017;71:80–94. https://doi.org/10.1016/j.ejca.2016.10.021. This review paper describes the impact of CABP and how adequate management of CABP can optimize the quality of life in cancer patients
Terpos E, Christoulas D, Gavriatopoulou M. Biology and treatment of myeloma related bone disease. Metabolism, 2017. https://doi.org/10.1016/j.metabol.2017.11.012, 2018.
Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med. 2000;6(5):521–8. https://doi.org/10.1038/74999.
Qin A, Cheng TS, Pavlos NJ, Lin Z, Dai KR, Zheng MH. V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int J Biochem Cell Biol. 2012;44(9):1422–35. https://doi.org/10.1016/j.biocel.2012.05.014.
• Maeda H, Kowada T, Kikuta J, Furuya M, Shirazaki M, Mizukami S, et al. Real-time intravital imaging of pH variation associated with osteoclast activity. Nat Chem Biol. 2016;12(8):579–85. https://doi.org/10.1038/nchembio.2096. This study introduces a new technique that allows quantitation of osteoclast activity and time-lapse imaging of its in vivo function during bone resorption using intravital imaging by two-photon excitation microscopy together with small fluorescent functional probes
Henriksen K, Sorensen MG, Jensen VK, Dziegiel MH, Nosjean O, Karsdal MA. Ion transporters involved in acidification of the resorption lacuna in osteoclasts. Calcif Tissue Int. 2008;83(3):230–42. https://doi.org/10.1007/s00223-008-9168-8.
Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone. 2006;39(5):1107–15. https://doi.org/10.1016/j.bone.2006.04.033.
Marelli S, Pace F. Rabeprazole for the treatment of acid-related disorders. Expert Rev Gastroenterol Hepatol. 2012;6(4):423–35. https://doi.org/10.1586/egh.12.18.
•• Peppicelli S, Andreucci E, Ruzzolini J, Laurenzana A, Margheri F, Fibbi G, et al. The acidic microenvironment as a possible niche of dormant tumor cells. Cell Mol Life Sci. 2017;74(15):2761–71. https://doi.org/10.1007/s00018-017-2496-y. This paper describes the critical role of acidity of cancer environment in stimulation of chemo- and radio-resistance, suppression of host immuno-surveilance, establishment of dormancy, and prognosis of cancer patients
Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–92. https://doi.org/10.1172/jci69741.
Halestrap AP. Monocarboxylic acid transport. Compr Physiol. 2013;3(4):1611–43. https://doi.org/10.1002/cphy.c130008.
Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145(1):11–9. https://doi.org/10.1016/j.neuroscience.2006.11.062.
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–23. https://doi.org/10.1016/j.cell.2011.02.018.
Gregory NS, Whitley PE, Sluka KA. Effect of intramuscular protons, lactate, and ATP on muscle hyperalgesia in rats. PLoS One. 2015;10(9):e0138576. https://doi.org/10.1371/journal.pone.0138576.
Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf). 2011;201(1):63–75. https://doi.org/10.1111/j.1748-1716.2010.02143.x.
Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128(3):549–58. https://doi.org/10.1016/j.pharmthera.2010.08.006.
Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport. 1998;9(6):1109–13.
Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun. 2005;337(1):349–54. https://doi.org/10.1016/j.bbrc.2005.09.054.
Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14(7):461–71. https://doi.org/10.1038/nrn3529.
Sun WH, Chen CC. Roles of proton-sensing receptors in the transition from acute to chronic pain. J Dent Res. 2016;95(2):135–42. https://doi.org/10.1177/0022034515618382.
Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106(3):229–39.
Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, et al. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161(4):950–60. https://doi.org/10.1111/j.1476-5381.2010.00918.x.
Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, et al. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23(7):1516–25. https://doi.org/10.1038/sj.emboj.7600177.
Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, et al. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010;68(1):61–72. https://doi.org/10.1016/j.neuron.2010.09.001.
Hsieh WS, Kung CC, Huang SL, Lin SC, Sun WH. TDAG8, TRPV1, and ASIC3 involved in establishing hyperalgesic priming in experimental rheumatoid arthritis. Sci Rep. 2017;7(1):8870. https://doi.org/10.1038/s41598-017-09200-6.
Qiu F, Wei X, Zhang S, Yuan W, Mi W. Increased expression of acid-sensing ion channel 3 within dorsal root ganglia in a rat model of bone cancer pain. Neuroreport. 2014;25(12):887–93. https://doi.org/10.1097/wnr.0000000000000182.
Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation. 2012;9:180. https://doi.org/10.1186/1742-2094-9-180.
Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, et al. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24(38):8310–21. https://doi.org/10.1523/jneurosci.2396-04.2004.
Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22(24):10662–70.
Marra S, Ferru-Clement R, Breuil V, Delaunay A, Christin M, Friend V, et al. Non-acidic activation of pain-related acid-sensing ion channel 3 by lipids. EMBO J. 2016;35(4):414–28. https://doi.org/10.15252/embj.201592335.
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13.
Lieben L, Carmeliet G. The involvement of TRP channels in bone homeostasis. Front Endocrinol (Lausanne). 2012;3:99. https://doi.org/10.3389/fendo.2012.00099.
Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25(12):3126–31. https://doi.org/10.1523/jneurosci.3815-04.2005.
Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience. 2007;148(2):560–72. https://doi.org/10.1016/j.neuroscience.2007.05.049.
Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth. 2009;102(2):251–8. https://doi.org/10.1093/bja/aen347.
Nakanishi M, Hata K, Nagayama T, Sakurai T, Nishisho T, Wakabayashi H, et al. Acid activation of Trpv1 leads to an up-regulation of calcitonin gene-related peptide expression in dorsal root ganglion neurons via the CaMK-CREB cascade: a potential mechanism of inflammatory pain. Mol Biol Cell. 2010;21(15):2568–77. https://doi.org/10.1091/mbc.E10-01-0049.
Xu Q, Zhang XM, Duan KZ, Gu XY, Han M, Liu BL, et al. Peripheral TGF-beta1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J Neurosci. 2013;33(49):19099–111. https://doi.org/10.1523/jneurosci.4852-12.2013.
Li Y, Cai J, Han Y, Xiao X, Meng XL, Su L, et al. Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur J Pain. 2014;18(6):774–84. https://doi.org/10.1002/j.1532-2149.2013.00420.x.
Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, et al. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell. 2014;157(5):1023–36. https://doi.org/10.1016/j.tem.2016.03.007.
• Riera CE, Dillin A. Emerging role of sensory perception in aging and metabolism. Trends Endocrinol Metab. 2016;27(5):294–303. https://doi.org/10.1016/j.cell.2014.03.051. This paper overviews the results of recent genetic studies using TRPV1 −/− mice that showed that sensory perception plays a role in influencing energy homeostasis and longevity
Moran MM. TRP channels as potential drug targets. Annu Rev Pharmacol Toxicol. 2018;58:309–30. https://doi.org/10.1146/annurev-pharmtox-010617-052832.
Jardin I, Lopez JJ, Diez R, Sanchez-Collado J, Cantonero C, Albarran L, et al. TRPs in pain sensation. Front Physiol. 2017;8:392. https://doi.org/10.3389/fphys.2017.00392.
Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–84. https://doi.org/10.1146/annurev-cellbio-101011-155833.
Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716(1–3):61–76. https://doi.org/10.1016/j.ejphar.2013.03.003.
Dalal S, Bruera E. Access to opioid analgesics and pain relief for patients with cancer. Nat Rev Clin Oncol. 2013;10(2):108–16. https://doi.org/10.1038/nrclinonc.2012.237.
Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260(1):76–87. https://doi.org/10.1111/j.1365-2796.2006.01667.x.
Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129(3):235–55. https://doi.org/10.1016/j.pain.2007.03.028.
Garami A, Ibrahim M, Gilbraith K, Khanna R, Pakai E, Miko A, et al. Transient receptor potential vanilloid 1 antagonists prevent anesthesia-induced hypothermia and decrease postincisional opioid dose requirements in rodents. Anesthesiology. 2017;127(5):813–23. https://doi.org/10.1097/aln.0000000000001812.
Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer. 2012;12(7):487–93. https://doi.org/10.1038/nrc3298.
Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y, et al. The a3 isoform vacuolar type H(+)-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res. 2011;9(7):845–55. https://doi.org/10.1158/1541-7786.mcr-10-0449.
McGuire C, Cotter K, Stransky L, Forgac M. Regulation of V-ATPase assembly and function of V-ATPases in tumor cell invasiveness. Biochim Biophys Acta. 2016;1857(8):1213–8. https://doi.org/10.1016/j.bbabio.2016.02.010.
Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27(31):5131–7. https://doi.org/10.1200/JCO.2009.22.4949.
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. https://doi.org/10.1126/science.1236361.
Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016;113(11):3078–83. https://doi.org/10.1073/pnas.1512603113.
Chatzistefanou I, Lubek J, Markou K, Ord RA. The role of perineural invasion in treatment decisions for oral cancer patients: a review of the literature. J Cranio-Maxillofac Surg. 2017;45(6):821–5. https://doi.org/10.1016/j.jcms.2017.02.022.
Yoneda T, Hiasa M, Nagata Y, Okui T, White FA. Acidic microenvironment and bone pain in cancer-colonized bone. Bonekey Rep. 2015;4:690. https://doi.org/10.1038/bonekey.2015.58.
Funding
This study is supported by the Project Development Team within the ICTSI NIH/NCRR (#TR000006) and start-up fund of Indiana University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Toshiyuki Yoneda, Masahiro Hiasa, and Tatsuo Okui declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Molecular Biology of Bone Metastasis
Rights and permissions
About this article
Cite this article
Yoneda, T., Hiasa, M. & Okui, T. Bone Pain Associated with Acidic Cancer Microenvironment. Curr Mol Bio Rep 4, 59–68 (2018). https://doi.org/10.1007/s40610-018-0089-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-018-0089-7