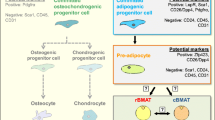
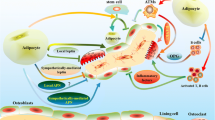
Abstract
Purpose of Review
Bone marrow adipocytes (BMAs) have distinct molecular properties and physiologic responses depending on their location within the skeleton.
Recent Findings
This concept was introduced in the 1970s and validated more recently in the contexts of cold exposure, sympathetic tone, hematopoiesis, diabetes, lactation, fasting, and caloric restriction.
Summary
In this brief review, we discuss the concept of regulated vs constitutive BMAs, explore their evolutionary and microenvironmental origins, define the site-specific molecular features of BMAs, and discuss the translational implications of the dual bone marrow adipose tissue hypothesis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6(6):7808. Defined regulated and constitutive bone marrow adipocytes.
Bathija A, Davis S, Trubowitz S. Marrow adipose tissue: response to erythropoiesis. Am J Hematol. 1978;5(4):315–21. https://doi.org/10.1002/ajh.2830050406.
• Scheller EL, Khandaker S, Learman BS, Cawthorn WP, Anderson LM, Pham HA, et al. Bone marrow adipocytes resist lipolysis and remodeling in response to β-adrenergic stimulation. Bone. 2018; https://doi.org/10.1016/j.bone.2018.01.016. Details molecular mechanisms underlying reduced responses of rBMAs and cBMAs to fasting and adrenergic cues.
Tavassoli M, Maniatis A, Crosby WH. Induction of sustained hemopoiesis in fatty marrow. Blood. 1974;43(1):33–8.
•• Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med. 1976;100(1):16–8. Original reference for the dual bone marrow adipose tissue hypothesis.
Bornstein S, Brown SA, Le PT, Wang X, DeMambro V, Horowitz MC, et al. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology. 2014;155(9):3516–26. https://doi.org/10.1210/en.2014-1083.
Cawthorn WP, Scheller EL. Editorial bone marrow adipose tissue: formation, function, and impact on health and disease. Front Endocrinol (Lausanne). 2017;8:112.
Hardouin P, Rharass T, Lucas S. Bone marrow adipose tissue: to be or not to be a typical adipose tissue? Front Endocrinol (Lausanne). 2016;7:85.
Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014 Apr;1311(1):14–30. https://doi.org/10.1111/nyas.12327.
Bigelow CL, Tavassoli M. Fatty involution of bone marrow in rabbits. Acta Anat (Basel). 1984;118(1):60–4. https://doi.org/10.1159/000145823.
Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, et al. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology. 2016;157(2):508–21. https://doi.org/10.1210/en.2015-1477.
Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skelet Radiol. 1985;14(1):10–9. https://doi.org/10.1007/BF00361188.
• Robles H, Park S, Joens M, Fitzpatrick J, Craft CS, Scheller EL. Characterization of the bone marrow adipocyte niche with three-dimensional electron microscopy. Bone. 2018; https://doi.org/10.1016/j.bone.2018.01.020. High-resolution visualization of the rBMA and cBMA niche.
Craft CS, Scheller EL. Evolution of the marrow adipose tissue microenvironment. Calcif Tissue Int. 2016;100(5):1–15.
Tavassoli M, Crosby WH. Bone marrow histogenesis: a comparison of fatty and red marrow. Science. 1970;169(3942):291–3. https://doi.org/10.1126/science.169.3942.291.
Tavassoli M. Hemopoiesis in ectopically implanted bone marrow. Kroc Found Ser. 1984;18:31–54.
Biermann A, Graf von Keyserlingk D. Ultrastructure of reticulum cells in the bone marrow. Acta Anat (Basel). 1978;100(1):34–43. https://doi.org/10.1159/000144879.
• Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771–784.e6. Definition of the BMA progenitor. https://doi.org/10.1016/j.stem.2017.02.009.
Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29(3):340–9. https://doi.org/10.1016/j.devcel.2014.03.013.
Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–68. https://doi.org/10.1016/j.stem.2014.06.008.
Scheller EL, Khoury B, Moller KL, Wee NK, Khandaker S, Kozloff KM, et al. Changes in skeletal integrity and marrow adiposity during high-fat diet and after weight loss. Front Endocrinol (Lausanne). 2016;7:102.
Emery JL, Follett GF. Regression of bone-marrow haemopoiesis from the terminal digits in the foetus and infant. Br J Haematol. 1964;10(4):485–9. https://doi.org/10.1111/j.1365-2141.1964.tb00725.x.
• Boyd AL, Reid JC, Salci KR, Aslostovar L, Benoit YD, Shapovalova Z, et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol. 2017;19(11):1336–47. The BMA supports myelo-erythropoiesis and hematopoietic equilibrium.
Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007 Oct 19;131(2):242–56. https://doi.org/10.1016/j.cell.2007.10.004.
Pond CM. The evolution of mammalian adipose tissue. In: Symonds ME, editor. Adipose Tissue Biology. New York: Springer New York; 2012. p. 227–69.
Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–75. https://doi.org/10.1016/j.cmet.2014.06.003.
Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25(9):2078–88. https://doi.org/10.1002/jbmr.82.
Tavassoli M. Differential response of bone marrow and extramedullary adipose cells to starvation. Experientia. 1974;30(4):424–5. https://doi.org/10.1007/BF01921701.
Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. 1979;6(3):191–8. https://doi.org/10.1002/ajh.2830060303.
Devlin MJ. Why does starvation make bones fat? Am J Hum Biol. 2011;23(5):577–85. https://doi.org/10.1002/ajhb.21202.
Mattiucci D, Maurizi G, Izzi V, Cenci L, Ciarlantini M, Mancini S, et al. Bone marrow adipocytes support hematopoietic stem cell survival. J Cell Physiol. 2018;233(2):1500–11. https://doi.org/10.1002/jcp.26037.
• Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891–903. https://doi.org/10.1038/ncb3570. BMAs secrete factors which support hematopoiesis.
Bigelow CL, Tavassoli M. Studies on conversion of yellow marrow to red marrow by using ectopic bone marrow implants. Exp Hematol. 1984;12(7):581–5.
Maniatis A, Tavassoli M, Crosby WH. Factors affecting the conversion of yellow to red marrow. Blood. 1971;37(5):581–6.
Tavassoli M. Marrow adipose cells. Ultrastructural and histochemical characterization. Arch Pathol. 1974;98(3):189–92.
Tavassoli M. Ultrastructural development of bone marrow adipose cell. Acta Anat (Basel). 1976;94(1):65–77. https://doi.org/10.1159/000144545.
Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab. 2016;27(6):392–403. https://doi.org/10.1016/j.tem.2016.03.016.
Sulston RJ, Cawthorn WP. Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm Mol Biol Clin Invest. 2016;28(1):21–38.
Tran MA, Lac DT, Berlan M, Lafontan M. Interplay of alpha-2 and beta adrenoceptors in the control of free fatty acid release from bone marrow adipose tissue. J Pharmacol Exp Ther. 1984;230(1):228–31.
Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014;289(24):16699–710. https://doi.org/10.1074/jbc.M114.547919.
Scheller EL, Burr AA, MacDougald OA, Cawthorn WP. Inside out: bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte. 2016;5(3):251–69. https://doi.org/10.1080/21623945.2016.1149269.
Blanchette-Mackie EJ, Scow RO. Lipolysis and lamellar structures in white adipose tissue of young rats: lipid movement in membranes. J Ultrastruct Res. 1981;77(3):295–318. https://doi.org/10.1016/S0022-5320(81)80026-3.
Qiang G, Whang Kong H, Xu S, Pham HA, Parlee SD, Burr AA, et al. Lipodystrophy and severe metabolic dysfunction in mice with adipose tissue-specific insulin receptor ablation. Mol Metab. 2016;5(7):480–90. https://doi.org/10.1016/j.molmet.2016.05.005.
Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28(8):1721–8. https://doi.org/10.1002/jbmr.1950.
Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22(2):279–85. https://doi.org/10.1002/jmri.20367.
Yu EW, Greenblatt L, Eajazi A, Torriani M, Bredella MA. Marrow adipose tissue composition in adults with morbid obesity. Bone. 2017;97:38–42. https://doi.org/10.1016/j.bone.2016.12.018.
Pino AM, Miranda M, Figueroa C, Rodríguez JP, Rosen CJ. Qualitative aspects of bone marrow adiposity in osteoporosis. Front Endocrinol (Lausanne). 2016;7:139.
Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12(1):212. https://doi.org/10.1186/1471-2164-12-212.
Liu LF, Shen WJ, Ueno M, Patel S, Azhar S, Kraemer FB. Age-related modulation of the effects of obesity on gene expression profiles of mouse bone marrow and epididymal adipocytes. PLoS One. 2013;8(8):e72367. https://doi.org/10.1371/journal.pone.0072367.
Poloni A, Maurizi G, Serrani F, Mancini S, Zingaretti MC, Frontini A, et al. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. 2013;41(6):558–566.e2. https://doi.org/10.1016/j.exphem.2013.02.005.
Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–52. https://doi.org/10.1016/j.bone.2011.06.016.
Lecka-Czernik B, Stechschulte LA, Czernik PJ, Sherman SB, Huang S, Krings A. Marrow adipose tissue: skeletal location, sexual dimorphism, and response to sex steroid deficiency. Front Endocrinol (Lausanne). 2017;8:188. https://doi.org/10.3389/fendo.2017.00188.
Tencerova M, Kassem M. The bone marrow-derived stromal cells: commitment and regulation of adipogenesis. Front Endocrinol (Lausanne). 2016;7:127.
Ge C, Zhao G, Li B, Li Y, Cawthorn WP, MacDougald OA, et al. Genetic inhibition of PPARγ S112 phosphorylation reduces bone formation and stimulates marrow adipogenesis. Bone. 2018;107:1–9. https://doi.org/10.1016/j.bone.2017.10.023.
• Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017;25(3):661–72. https://doi.org/10.1016/j.cmet.2017.01.001. BMAs can secrete bone-modifying factors including RANKL
Liu LF, Shen WJ, Zhang ZH, Wang LJ, Kraemer FB. Adipocytes decrease Runx2 expression in osteoblastic cells: roles of PPARγ and adiponectin. J Cell Physiol. 2010;225(3):837–45. https://doi.org/10.1002/jcp.22291.
•• Lu W, Weng W, Zhu Q, Zhai Y, Wan Y, Liu H, et al. Small bone marrow adipocytes predict poor prognosis in acute myeloid leukemia. Haematologica. 2018;103(1):e21–4. Utilization of lipid from BMAs may predict poor clinical prognosis of malignancy.
Funding
This work was funded by the National Institutes of Health including a pilot grant from the Washington University Musculoskeletal Research Center P30-AR057235 (C.S.C.), R24-DK092759 (O.A.M.), and R00-DE024178 (E.L.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Clarissa S. Craft, Ziru Li, Ormond A. MacDougald, and Erica L. Scheller each declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Molecular Biology of Bone Marrow Fat Adiposity
Rights and permissions
About this article
Cite this article
Craft, C.S., Li, Z., MacDougald, O.A. et al. Molecular Differences Between Subtypes of Bone Marrow Adipocytes. Curr Mol Bio Rep 4, 16–23 (2018). https://doi.org/10.1007/s40610-018-0087-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-018-0087-9