Abstract
Purpose of Review
Copper, zinc, iron, and manganese are essential micronutrients for all living organisms. Microbial pathogens must acquire these elements from their host. Through a process termed nutritional immunity, animal hosts seek to withhold these vital nutrients from the microbe and the competition for metals can influence survival outcomes during infection. Much is known about the battle for iron, copper, and zinc during fungal infections, but a picture is just now beginning to emerge for manganese.
Recent Findings
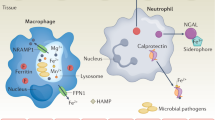
Pathogenic fungi utilize manganese for antioxidant defense, cell wall construction, morphogenesis, and survival in animal and plant hosts. The animal host can limit manganese availability for invading fungi at the macrophage, neutrophil, and whole tissue levels.
Summary
Here, we review the role of manganese as an essential nutrient for pathogenic fungi and the ways an animal host can withhold this vital metal from infectious fungi of clinical and agricultural importance.


Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460(7257):823–30. https://doi.org/10.1038/nature08300nature08300.
• Murdoch CC, Skaar EP. Nutritional immunity: the battle for nutrient metals at the host-pathogen interface. Nat Rev Microbiol. 2022;20(11):657–70. https://doi.org/10.1038/s41579-022-00745-6. Comprehensive review on nutritional immunity involving metals including manganese.
Wilson D. Chapter two - the role of zinc in the pathogenicity of human fungal pathogens. In: Gadd GM, Sariaslani S, editors. Advances in applied microbiology. Academic Press; 2021. p. 35–61.
Gupta M, Outten CE. Iron-sulfur cluster signaling: the common thread in fungal iron regulation. Curr Opin Chem Biol. 2020;55:189–201. https://doi.org/10.1016/j.cbpa.2020.02.008.
Smith AD, Logeman BL, Thiele DJ. Copper acquisition and utilization in fungi. Annu Rev Microbiol. 2017;71:597–623. https://doi.org/10.1146/annurev-micro-030117-020444.
Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, et al. Superoxide dismutases and superoxide reductases. Chem Rev. 2014;114(7):3854–918. https://doi.org/10.1021/cr4005296.
Culbertson EM, Bruno VM, Cormack BP, Culotta VC. Expanded role of the Cu-sensing transcription factor Mac1p in Candida albicans. Mol Microbiol. 2020;114(6):1006–18. https://doi.org/10.1111/mmi.14591.
Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112(38):E5336–42. https://doi.org/10.1073/pnas.1513447112.
Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Met Ions Life Sci. 2013;13:199–227. https://doi.org/10.1007/978-94-007-7500-8_7.
Bates S, Hughes HB, Munro CA, Thomas WP, MacCallum DM, Bertram G, et al. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem. 2006;281(1):90–8. https://doi.org/10.1074/jbc.M510360200.
Bai C, Xu XL, Chan FY, Lee RT, Wang Y. MNN5 encodes an iron-regulated alpha-1,2-mannosyltransferase important for protein glycosylation, cell wall integrity, morphogenesis, and virulence in Candida albicans. Eukaryot Cell. 2006;5(2):238–47. https://doi.org/10.1128/ec.5.2.238-247.2006.
Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Essential metals in health and disease. Chem Biol Interact. 2022;367: 110173. https://doi.org/10.1016/j.cbi.2022.110173.
•• Nicastro R, Gaillard H, Zarzuela L, Péli-Gulli MP, Fernández-García E, Tomé M, et al. Manganese is a physiologically relevant TORC1 activator in yeast and mammals. Elife. 2022;11. https://doi.org/10.7554/eLife.80497. First to show a role for Mn in fungal TOR signaling.
• Cellier MFM. Nramp: Deprive and conquer? Front Cell Dev Biol. 2022;10:988866. https://doi.org/10.3389/fcell.2022.988866. Role for NRAMP transporters in nutritional immunity including manganese.
West AH, Clark DJ, Martin J, Neupert W, Hart FU, Horwich AL. Two related genes encoding extremely hydrophobic proteins suppress a lethal mutation in the yeast mitochondrial processing enhancing protein. J Biol Chem. 1992;267:24625–33.
Liu XF, Culotta VC. Post-translational control of Nramp metal transport in yeast: role of metal ions and the BSD2 gene. J Biol Chem. 1999;274:4863–8.
Liu XF, Supek F, Nelson N, Culotta VC. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J Biol Chem. 1997;272:11763–9.
Luk E, Culotta VC. Manganese superoxide dismutase in S. cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transproter, Smf2p. J Biol Chem. 2001;276:47556–62.
Liu XF, Culotta VC. Mutational analysis of Saccharomyces cerevisiae Smf1p, a member of the Nramp family of metal transporters. J Mol Biol. 1999;289(4):885–91. https://doi.org/10.1006/jmbi.1999.2815.
Jensen LT, Ajua-Alemanji M, Culotta VC. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J Biol Chem. 2003;278:42036–40.
Trilisenko L, Zvonarev A, Valiakhmetov A, Penin AA, Eliseeva IA, Ostroumov V, et al. The reduced level of inorganic polyphosphate mobilizes antioxidant and manganese-resistance systems in Saccharomyces cerevisiae. Cells. 2019;8(5). https://doi.org/10.3390/cells8050461.
Stimpson HE, Lewis MJ, Pelham HR. Transferrin receptor-like proteins control the degradation of a yeast metal transporter. Embo j. 2006;25(4):662–72. https://doi.org/10.1038/sj.emboj.7600984.
Sullivan JA, Lewis MJ, Nikko E, Pelham HR. Multiple interactions drive adaptor-mediated recruitment of the ubiquitin ligase rsp5 to membrane proteins in vivo and in vitro. Mol Biol Cell. 2007;18(7):2429–40. https://doi.org/10.1091/mbc.e07-01-0011.
Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9(12):1216–21. https://doi.org/10.1038/embor.2008.199.
Nikko E, Pelham HR. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic. 2009;10(12):1856–67. https://doi.org/10.1111/j.1600-0854.2009.00990.x.
Jensen LT, Carroll MC, Hall MD, Harvey CJ, Beese SE, Culotta VC. Down-regulation of a manganese transporter in the face of metal toxicity. Mol Biol Cell. 2009;20(12):2810–9. https://doi.org/10.1091/mbc.E08-10-1084.
Durr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, et al. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Molec Biol Cell. 1998;9:1149–62.
Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, et al. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca+2-ATPase family. Cell. 1989;58:133–45.
Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol. 1995;15:1382–8.
Thines L, Deschamps A, Sengottaiyan P, Savel O, Stribny J, Morsomme P. The yeast protein Gdt1p transports Mn(2+) ions and thereby regulates manganese homeostasis in the Golgi. J Biol Chem. 2018;293(21):8048–55. https://doi.org/10.1074/jbc.RA118.002324.
• Deschamps A, Thines L, Colinet AS, Stribny J, Morsomme P. The yeast Gdt1 protein mediates the exchange of H(+) for Ca(2+) and Mn(2+) influencing the Golgi pH. J Biol Chem. 2023;299(5):104628. https://doi.org/10.1016/j.jbc.2023.104628. Description of the mechanism of action of the Golgi Mn and Ca transporter GDT1.
Luk E, Carroll M, Baker M, Culotta VC. Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc Natl Acad Sci USA. 2003;100:10353–7.
Yang M, Cobine PA, Molik S, Naranuntarat A, Lill R, Winge DR, et al. The effects of mitochondrial iron homeostasis on cofactor specificity of superoxide dismutase 2. EMBO J. 2006;25:1775–83.
Naranuntarat A, Jensen LT, Pazicni S, Penner-Hahn JE, Culotta VC. The interaction of mitochondrial iron with manganese superoxide dismutase. J Biol Chem. 2009;284(34):22633–40. https://doi.org/10.1074/jbc.M109.026773.
Wolff NA, Garrick MD, Zhao L, Garrick LM, Ghio AJ, Thévenod F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci Rep. 2018;8(1):211. https://doi.org/10.1038/s41598-017-18584-4.
Wolff NA, Garrick LM, Zhao L, Garrick MD, Thévenod F. Mitochondria represent another locale for the divalent metal transporter 1 (DMT1). Channels (Austin). 2014;8(5):458–66. https://doi.org/10.4161/19336950.2014.956564.
• Bozzi AT, Gaudet R. Molecular mechanism of Nramp-family transition metal transport. J Mol Biol. 2021;433(16):166991. https://doi.org/10.1016/j.jmb.2021.166991. Review showing functions of NRAMP transporters including interactions with the mitochondria and delivery of metals to mitochondria including Mn and Fe.
Diss L, Blaudez D, Gelhaye E, Chalot M. Genome-wide analysis of fungal manganese transporters, with an emphasis on Phanerochaete chrysosporium. Environ Microbiol Rep. 2011;3(3):367–82. https://doi.org/10.1111/j.1758-2229.2010.00235.x.
Agranoff D, Collins L, Kehres D, Harrison T, Maguire M, Krishna S. The Nramp orthologue of Cryptococcus neoformans is a pH-dependent transporter of manganese, iron, cobalt and nickel. Biochem J. 2005;385(Pt 1):225–32. https://doi.org/10.1042/bj20040836.
Fejes B, Ouedraogo JP, Fekete E, Sándor E, Flipphi M, Soós Á, et al. The effects of external Mn(2+) concentration on hyphal morphology and citric acid production are mediated primarily by the NRAMP-family transporter DmtA in Aspergillus niger. Microb Cell Fact. 2020;19(1):17. https://doi.org/10.1186/s12934-020-1286-7.
Fan J, Zhang H, Li Y, Chen Z, Chen T, Zeng B, et al. Identification and characterization of Nramp transporter AoNramp1 in Aspergillus oryzae. 3 Biotech. 2021;11(10):452. https://doi.org/10.1007/s13205-021-02998-z.
Xu N, Dong Y, Cheng X, Yu Q, Qian K, Mao J, et al. Cellular iron homeostasis mediated by the Mrs4-Ccc1-Smf3 pathway is essential for mitochondrial function, morphogenesis and virulence in Candida albicans. Biochim Biophys Acta. 2014;1843(3):629–39. https://doi.org/10.1016/j.bbamcr.2013.12.009.
•• Wildeman AS, Patel NK, Cormack BP, Culotta VC. The role of manganese in morphogenesis and pathogenesis of the opportunistic fungal pathogen Candida albicans. PLoS Pathog. 2023;19(6):e1011478. https://doi.org/10.1371/journal.ppat.1011478. First evidence showing a role for a Mn-specific fungal NRAMP in fungal pathogenesis of a mammalian host and a host response involving lowering of kidney Mn.
• Henry M, Khemiri I, Tebbji F, Abu-Helu R, Vincent AT, Sellam A. Manganese homeostasis modulates fungal virulence and stress tolerance in Candida albicans. bioRxiv. 2023:2023.10.12.562042. https://doi.org/10.1101/2023.10.12.562042. Fungal Mn NRAMP transporter important for pathogenesis in an insect model.
Evangelinos M, Martzoukou O, Chorozian K, Amillis S, Diallinas G. BsdA(Bsd2) -dependent vacuolar turnover of a misfolded version of the UapA transporter along the secretory pathway: prominent role of selective autophagy. Mol Microbiol. 2016;100(5):893–911. https://doi.org/10.1111/mmi.13358.
Liu NN, Uppuluri P, Broggi A, Besold A, Ryman K, Kambara H, et al. Intersection of phosphate transport, oxidative stress and TOR signalling in Candida albicans virulence. PLoS Pathog. 2018;14(7): e1007076. https://doi.org/10.1371/journal.ppat.1007076.
Bates S, MacCallum DM, Bertram G, Munro CA, Hughes HB, Buurman ET, et al. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J Biol Chem. 2005;280(24):23408–15.
•• Qu S, Chi SD, He ZM. The development of Aspergillus flavus and biosynthesis of aflatoxin B1 are regulated by the Golgi-localized Mn(2+) transporter Pmr1. J Agric Food Chem. 2024;72(2):1276–91. https://doi.org/10.1021/acs.jafc.3c06964. Evidence supporting a role for Golgi Mn in morphogenesis and pathogenesis in a filamentous fungus.
• Wu C, Guo Z, Zhang M, Chen H, Peng M, Abubakar YS, et al. Golgi-localized calcium/manganese transporters FgGdt1 and FgPmr1 regulate fungal development and virulence by maintaining Ca2+ and Mn2+ homeostasis in Fusarium graminearum. Environ Microbiol. 2022;24(10):4623–40. https://doi.org/10.1111/1462-2920.16128. Golgi Mn and Ca transporter are important for fungal morphogenesis and pathogenesis.
Briard B, Fontaine T, Kanneganti TD, Gow NAR, Papon N. Fungal cell wall components modulate our immune system. Cell Surf. 2021;7: 100067. https://doi.org/10.1016/j.tcsw.2021.100067.Thefungalcellwallplaysaroleinimmunerecognition.
Yadav B, Mora-Montes HM, Wagener J, Cunningham I, West L, Haynes K, et al. Differences in fungal immune recognition by monocytes and macrophages: N-mannan can be a shield or activator of immune recognition. Cell Surf. 2020;6: 100042. https://doi.org/10.1016/j.tcsw.2020.100042.
Hopke A, Brown AJP, Hall RA, Wheeler RT. Dynamic fungal cell wall architecture in stress adaptation and immune evasion. Trends Microbiol. 2018;26(4):284–95. https://doi.org/10.1016/j.tim.2018.01.007.
Pinchai N, Juvvadi PR, Fortwendel JR, Perfect BZ, Rogg LE, Asfaw YG, et al. The Aspergillus fumigatus P-type Golgi apparatus Ca2+/Mn2+ ATPase PmrA is involved in cation homeostasis and cell wall integrity but is not essential for pathogenesis. Eukaryot Cell. 2010;9(3):472–6. https://doi.org/10.1128/ec.00378-09.
Nguyen QB, Kadotani N, Kasahara S, Tosa Y, Mayama S, Nakayashiki H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol Microbiol. 2008;68(6):1348–65. https://doi.org/10.1111/j.1365-2958.2008.06242.x.
López-Lorca VM, Molina-Luzón MJ, Ferrol N. Characterization of the NRAMP gene family in the arbuscular mycorrhizal fungus Rhizophagus irregularis. J Fungi (Basel). 2022;8(6). https://doi.org/10.3390/jof8060592.
Shafeeq S, Pannanusorn S, Elsharabasy Y, Ramírez-Zavala B, Morschhäuser J, Römling U. Impact of manganese on biofilm formation and cell morphology of Candida parapsilosis clinical isolates with different biofilm forming abilities. FEMS Yeast Res. 2019;19(6). https://doi.org/10.1093/femsyr/foz057.
Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192(9):1237–48. https://doi.org/10.1084/jem.192.9.1237.
Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, et al. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182(3):655–66. https://doi.org/10.1084/jem.182.3.655.
Blasi E, Colombari B, Mucci A, Cossarizza A, Radzioch D, Boelaert JR, et al. Nramp1 gene affects selective early steps in macrophage-mediated anti-cryptococcal defense. Med Microbiol Immunol. 2001;189(4):209–16. https://doi.org/10.1007/s004300100066.
Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10(8):525–37. https://doi.org/10.1038/nrmicro2836.
Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol. 2015;11(10):765–71. https://doi.org/10.1038/nchembio.1891.
Brophy MB, Nakashige TG, Gaillard A, Nolan EM. Contributions of the S100A9 C-terminal tail to high-affinity Mn(II) chelation by the host-defense protein human calprotectin. J Am Chem Soc. 2013;135(47):17804–17. https://doi.org/10.1021/ja407147d.
Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A. 2013;110(10):3841–6. https://doi.org/10.1073/pnas.1220341110.
Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, et al. Role of calprotectin in withholding zinc and copper from Candida albicans. Infect Immun. 2018;86(2). https://doi.org/10.1128/iai.00779-17.
Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10): e1000639. https://doi.org/10.1371/journal.ppat.1000639.
Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus CS, Skaar EP, et al. Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J Immunol. 2016;196(1):336–44. https://doi.org/10.4049/jimmunol.1502037.
•• Sunuwar L, Tomar V, Wildeman A, Culotta V, Melia J. Hepatobiliary manganese homeostasis is dynamic in the setting of inflammation or infection in mice. Faseb j. 2023;37(9):e23123. https://doi.org/10.1096/fj.202300539R. First to show a host response involving loss of liver Mn during fungal infection.
• Hall SC, Smith DR, Dyavar SR, Wyatt TA, Samuelson DR, Bailey KL, et al. Critical role of zinc transporter (ZIP8) in myeloid innate immune cell function and the host response against bacterial pneumonia. J Immunol. 2021;207(5):1357–70. https://doi.org/10.4049/jimmunol.2001395. A mammalian Mn (and Zn) transporter that responds to infection and inflammation.
Sunuwar L, Frkatović A, Sharapov S, Wang Q, Neu HM, Wu X, et al. Pleiotropic ZIP8 A391T implicates abnormal manganese homeostasis in complex human disease. JCI Insight. 2020;5(20). https://doi.org/10.1172/jci.insight.140978.
Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol. 2009;71(1):240–52.
Zacchi LF, Gomez-Raja J, Davis DA. Mds3 regulates morphogenesis in Candida albicans through the TOR pathway. Mol Cell Biol. 2010;30(14):3695–710. https://doi.org/10.1128/mcb.01540-09.
Irving H, Williams RJP. 637. The stability of transition-metal complexes. J Chem Soc. 1953;3192–210. https://doi.org/10.1039/JR9530003192.
Almeida F, Rodrigues ML, Coelho C. The still underestimated problem of fungal diseases worldwide. Front Microbiol. 2019;10:214. https://doi.org/10.3389/fmicb.2019.00214.
Denning DW, Bromley MJ. Infectious disease. How to bolster the antifungal pipeline. Science. 2015;347(6229):1414–6. https://doi.org/10.1126/science.aaa6097.
Casadevall A, Kontoyiannis DP, Robert V. On the emergence of Candida auris: climate change, azoles, swamps, and birds. mBio. 2019;10(4). https://doi.org/10.1128/mBio.01397-19.
Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10): e1008921. https://doi.org/10.1371/journal.ppat.1008921.
Dixit A, Carroll SF, Qureshi ST. Cryptococcus gattii: an emerging cause of fungal disease in North America. Interdiscip Perspect Infect Dis. 2009;2009: 840452. https://doi.org/10.1155/2009/840452.
Savary S, Ficke A, Aubertot J-N, Hollier C. Crop losses due to diseases and their implications for global food production losses and food security. Food Security. 2012;4(4):519–37. https://doi.org/10.1007/s12571-012-0200-5.
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186–94. https://doi.org/10.1038/nature10947.
Funding
ASW and VCC are supported by the National Institutes of Health Grants R21AI154726 and R35GM136644.
Author information
Authors and Affiliations
Contributions
AW and VC wrote the main text and VC prepared figures. Both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies performed by the authors that were reported to involve animals have been published and were compliant with Johns Hopkins Institutional Animal Care and Use Committee and with the guidelines of the Animal Welfare Act and Public Health Service Policy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Fungal Pathogenesis
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wildeman, A.S., Culotta, V.C. Nutritional Immunity and Fungal Pathogens: A New Role for Manganese. Curr Clin Micro Rpt 11, 70–78 (2024). https://doi.org/10.1007/s40588-024-00222-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-024-00222-z