Abstract
Purpose of Review
This review serves to highlight the cross-kingdom interactions that can occur within the human oral cavity between fungus Candida albicans and oral bacteria, and their impact on the delicate balance between oral health and disease.
Recent Findings
A growing number of physical, chemical, and metabolic networks have been identified that underpin these cross-kingdom interactions. Moreover, these partnerships are often synergistic and can modulate microbial burden or virulence. This, in turn, can drive the onset or progression of oral diseases such as dental caries, periodontitis, denture-associated stomatitis, and oral cancer.
Summary
The impact of cross-kingdom interactions on the cellular, biochemical, and communal composition of oral microbial biofilms is increasingly clear. With growing insight into these processes at the molecular level, so this knowledge can be used to better inform the development of novel strategies to manipulate the oral microbiota to promote oral health and combat oral disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oral cavity is defined as the three-dimensional space that extends anteroposteriorly from the lips to the faucial pillars, from the hard palate to the floor of the mouth dorsoventrally, and is bounded by the cheeks laterally. The oral cavity connects the external environment with the digestive and respiratory tracts and performs a wide spectrum of functions, ranging from mastication and deglutition through to taste sensation and speech. Important anatomical structures found within the oral cavity include the teeth, tongue, and hard and soft palates. Together with other foreign materials (e.g. dentures, fillings, orthodontic appliances, crowns, and bridges), these surfaces provide a range of sites that, in turn, support colonisation by diverse communities of microorganisms known collectively as the oral microbiome [1•]. As the second most diverse microbiome within the human body, members of the kingdoms Monera, Protista, and Fungi are represented in various oral habitats. To date, over 700 different species of bacteria, together with fungi, viruses, archaea, and protozoa, have been found within the oral cavity [2].
To optimise their existence within the distinct and unique micro-niches of the oral cavity, oral microbes have principally evolved a communal lifestyle in which they form complex, three-dimensional, and hierarchical bio-communal units called ‘biofilms’. These biofilms are complex micro-ecosystems of surface-attached microorganisms that are embedded within a self-produced exopolymeric matrix [3]. Biofilms serve to provide a protective niche for their microbial inhabitants, and the role of oral biofilms in promoting both oral health and disease is well established [4]. In addition, strong evidence of the correlations between dysbiosis of the oral microbiome and various systemic diseases is emerging [5•]. Within oral biofilms, the microbial inhabitants develop complex and unique physical, chemical, and metabolic interactions. These can occur between microbes of the same species (intraspecies interactions), different species (interspecies interactions), or different kingdoms (cross-kingdom interactions). Extensive crosstalk also occurs between the oral microbiota and the host [3]. This review highlights our current understanding of cross-kingdom interactions within the human oral cavity between oral bacteria and the predominant fungus, Candida albicans, and their impact on oral disease (Table 1).
Candida albicans and Oral Disease
Alongside the 700 + species of bacteria that can inhabit the human oral cavity, over 100 species of fungi have also been reported [6]. It is perhaps unsurprising, therefore, that complex interactions between these two kingdoms are evident. Whilst the associations of various bacterial species with oral disease are well established, the role of fungi in oral infections has been best studied in relation to Candida species. Other fungal genera such as Saccharomycetales, Cladosporium, Aureobasidium, Aspergillus, Fusarium, and Cryptococcus can also comprise the oral mycobiome; however, their association with oral disease or interactions with resident oral bacteria are less well known [6]. C. albicans is a dimorphic, opportunistic fungus that colonises the oral cavities of over half the human population [7]. Cross-kingdom interactions of this fungus with several bacterial species have been associated with the onset or progression of various oral diseases, prominent examples of which are outlined below.
Cross-kingdom Interactions and Oral Disease
Dental Caries
C. albicans is considered a major cariogenic fungal pathogen and evidence suggests that, together with Streptococcus mutans, C. albicans can synergistically promote the onset and progression of dental caries of varying severity [8, 9]. Extracellular polymeric substances (EPS) synthesised by the bacterium promote fungal growth and metabolism, which in turn creates a vicious cycle by stimulating further bacterial growth and EPS production [10]. By associating with S. mutans, C. albicans benefits from the colonisation advantage conferred by the low pH conditions that result from S. mutans acid synthesis. It is the maintenance of these acidic conditions that also causes severe enamel demineralisation and hence caries progression [9]. There is a substantial body of evidence to indicate a correlation between biofilms of C. albicans and cariogenic bacteria and the severity of root caries. In particular, candidal hyphae have been found to aid colonisation of cariogenic bacteria by forming filamentous networks or corncob configurations with other non-mutans streptococci [11]. C. albicans is also frequently co-isolated with other key bacterial pathogens of root caries such as Actinomyces viscosus. These two microorganisms appear to form robust dual-species biofilms that are acidogenic and cause severe damage to hydroxyapatite in vitro. Again, this suggests a synergistic partnership that can exacerbate the initiation and progression of dental caries [12].
Periodontal Disease
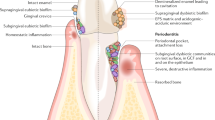
The link between fungal and bacterial interactions and the pathogenesis of periodontal diseases is well established. C. albicans has been co-located with periodontopathogens such as Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola in sites of periodontitis. Importantly, periodontal sites from which the fungus was co-isolated with either T. forsythia or T. denticola appeared to display a greater surface area of inflammation, suggesting a synergism between the bacteria and fungus triggering periodontal tissue damage [13]. Of all the periodontal bacterial pathogens, the interactions between P. gingivalis and C. albicans have been explored in most depth. These two microbes can engage via direct physical contact [14, 15], whilst host-derived heme, a key source of iron for both microorganisms, enhances the pathogenic potential of P. gingivalis in the presence of C. albicans. This leads to increased invasion of the gingival tissues, greater destruction of periodontal tissues, and a delay in wound healing [16, 17]. This interplay likely also explains the co-isolation of P. gingivalis and C. albicans from deep periodontal pockets in mouse models and their association with active bleeding and chronic infection [17, 18].
Denture-Associated Stomatitis
Candida species are the principal pathogens that cause denture-associated stomatitis. However, various bacteria have been shown to modulate Candida pathogenicity as part of the disease process. An early in vitro study noted a synergism between C. albicans and oral bacteria Actinomyces oris and S. oralis grown on denture material, in which the bacteria stimulated C. albicans hyphae formation and coaggregated with them, whilst C. albicans enhanced bacterial cell numbers [19]. Similarly, a recent study has identified a potential synergism between C. albicans and three acidogenic bacterial genera — Lactobacillus, Scardovia, and Bifidobacterium — in dental plaque samples isolated from dentures. A negative correlation was also noted for C. albicans and some periodontal pathogens (Porphyromonas, Catonella, Capnocytophaga, Bulleidia), highlighting the complexity of cross-kingdom interactions occurring on the denture surface [20].
Oral Cancer
C. albicans is considered a potential major contributor to oral cancer development through its ability to trigger inflammation and induce Th17 responses, and to produce hydrolytic enzymes and carcinogenic acetaldehyde [21, 22]. An in vitro biofilm study noted that the interactions of C. albicans with Actinomyces naeslundii and S. mutans modulated cancer cell phenotype by increasing the adhesion of oral squamous cell carcinoma cells to the tissue extracellular matrix. This was accompanied by the elevated expression of proinflammatory cytokines, potentially generating a tumour-promoting effect [23]. Likewise, secretory components from C. albicans and S. aureus dual-species biofilms were shown to alter the expression of proto-oncogenes in normal and neoplastic oral epithelial cells, thus disturbing the cell cycle and potentially promoting oral carcinogenesis [24]. Using animal models, dysbiosis as a result of chemotherapy has been associated with overgrowth of C. albicans in the presence of Enterococcus faecalis. Again, this has been linked with an increase in fungal virulence that may enhance disruption of the mucosal barrier by the release of proteolytic enzymes [25]. Candida species and various bacteria (e.g. P. gingivalis, Fusobacterium nucleatum, Streptococcus species) have also been frequently co-detected in oral squamous cell carcinomas [26•]. In this instance, however, strong clinical evidence for a causative effect has yet to emerge.
Mechanistic Basis of Cross-kingdom Interactions
Physical Interactions
Early (or primary) colonisers initiate the accretion of polymicrobial biofilms on oral surfaces. These bacteria are mainly streptococci (e.g. S. sanguinis, S. mitis, S. oralis) and Actinomyces species, and are capable of directly binding to components of the salivary pellicle that coats the soft and hard tissues of the oral cavity. These early colonisers can then support the acquisition of late (or secondary) colonisers, which bind to protein or polysaccharide receptors on the surface of primary colonisers in a process known as coadhesion [27]. As a late coloniser, C. albicans can physically interact with several oral bacteria, thereby enabling C. albicans to be retained within the oral cavity and evade the flushing forces of saliva [28, 29]. This can be visualised in dental plaque with the formation of corncob structures, in which the hyphal filaments of C. albicans are decorated by bound bacteria including mitis group streptococci and P. gingivalis [11, 30,51,32]. At the molecular level, C. albicans hypha-specific cell wall adhesins, Als3 and Hwp1, have been shown to facilitate binding to S. gordonii and S. oralis. Moreover, for S. gordonii, antigen I/II family polypeptide SspB has been identified as the cognate ligand for Als3 [30, 33]. Given the widespread expression of this adhesin family among streptococci and the proposed capacity for Als3 to bind a broad range of peptide ligands, this likely represents a common mechanism by which biofilms between oral streptococci and C. albicans are supported [32, 34]. Als3 also facilitates candidal interactions with periodontopathogen P. gingivalis by specifically binding InlJ [15]. In both instances, these cross-kingdom interactions have implications for oral disease outcome. For example, S. oralis and C. albicans synergise in dual-species biofilms to exacerbate mucosal tissue invasion and disruption of epithelial barriers to promote the development of oral thrush lesions [35]. Likewise, C. albicans enhances the expression of key virulence factors and tissue invasion by P. gingivalis [36], effects that could elevate the risk of periodontal disease progression. Physical interactions between C. albicans and E. faecalis have also been noted in recurrent endodontic infections, and co-infection has been shown to augment bone resorption and inflammation, resulting in more extensive periapical lesions. Again. E. faecalis has been shown to adhere to C. albicans hyphae as well as yeast cells within infected tooth root canals and dentinal tubules but in this instance, the precise ligand-receptor interactions have yet to be defined [37, 38].
Recently, it has also been revealed that cross-kingdom physical interactions that occur within saliva can influence subsequent colonisation of the tooth surface and dental caries risk. Specifically, clusters of S. mutans have been shown to attach to networks of C. albicans hyphae and glucan EPS within saliva to form a structured unit that displays enhanced attachment to the tooth surface and proliferation [39•]. Furthermore, these assemblages promote microbial motility and spreading, ultimately resulting in the formation of a biofilm super-structure, the synergistic effects of which are elevated demineralisation of the tooth surface and progression of carious lesions.
Chemical Signalling
Complementing direct physical contact between microbes, a second major mechanism that underpins cross-kingdom interactions within the oral cavity is chemical crosstalk. In many instances, it is the quorum-sensing (QS) systems that direct co-ordinated population responses that are exploited for this purpose. For oral streptococci, despite differences in the precise amino acid sequence, competence stimulating peptide (CSP) from several species, including S. mutans and S. gordonii, has been shown to impair candidal hyphae formation [40, 41]. Likewise, gelatinase biosynthesis-activating pheromone (GBAP), a QS molecule (QSM) associated with the Fsr QS system of E. faecalis, inhibited C. albicans filamentation in a Caenorhabditis elegans infection model [42, 43]. Autoinducer-2 (AI-2), the ‘universal’ QSM, has also been shown to influence candidal morphogenesis but in this instance, divergent outcomes have been reported. AI-2 from S. gordonii was found to stimulate hyphae formation by C. albicans by activating mitogen-activated protein kinase Cek1p, inhibiting Mkc1p and activating Hog1p [42, 44]. By contrast, the inverse effect was mediated by AI-2 from Aggregatibacter actinomycetemcomitans [45, 46]. Other, non-QS signals, can also impact C. albicans, including trans-2-decenoic acid from S. mutans that, again, has capacity to impair hyphae formation [47]. Such interactions are not, however, unidirectional and chemical signals released by C. albicans can also modulate oral bacteria. For example, farnesol has been shown to promote growth of S. mutans, although when concentrations exceed 100 µM, this effect is reversed [48, 49]. From the examples provided here, it is clear that the manipulation of C. albicans hyphae development is a key outcome of chemical crosstalk within the oral cavity. This may represent a common strategy via which the resident microbiota functions to keep C. albicans levels in check and thereby benefit from the presence of hyphae as attachment sites, whilst avoiding possible exclusion due to candidal outgrowth. Given the associations of C. albicans filamentation with tissue penetration and invasive disease, these also represent key mechanisms via which oral bacteria may influence the balance between oral health and disease.
Metabolic Interactions
Alongside crosstalk via the exchange of chemical signals, the oral microbiota has been shown to use metabolite-mediated cooperation (MMC) strategies to optimise their persistence within oral niches. MMC is based on the concept of the production and secretion of primary or secondary metabolites for use by the surrounding microbial community. These metabolic partnerships can be broadly divided into two categories: (i) cross-feeding, in which one microbial species synthesises and secretes metabolites for the benefit of their partner microorganism(s); or (ii) syntrophic strategy, a process by which one species partially catabolises complex molecules for energy synthesis, with the resultant partially degraded compounds then further catalysed by other microorganisms [50]. Again, within the oral cavity, many such metabolic interactions impact C. albicans morphogenesis. For example, peptidoglycan fragments (muropeptides) derived from the bacterial cell wall, the release of hydrogen peroxide as a result of aerobic metabolism and excreted nutrient by-products from oral streptococci such as S. gordonii have all been shown to promote hyphae formation [44, 51, 52]. By contrast, S. mutans produces a secondary metabolite known as mutanobactin A that impairs filamentation [53].
There are also changes within the local environment that occur as a result of microbial metabolism that can significantly impact the composition of the polymicrobial community within that ecological niche. A key example is oxygen availability. Aerobes utilise oxygen from their immediate environment, thereby establishing a low oxygen tension that favours the persistence of obligate anaerobes [50]. Consequently, the reduction in oxygen tension in the microenvironment due to aerobic metabolism by C. albicans has been shown to allow strict anaerobes such as Veillonella, Prevotella, and Fusobacterium to thrive in the otherwise oxygen-rich environment of the oral cavity [54]. Likewise, periodontal pathogens P. gingivalis and T. forsythia benefit from oxygen depletion in biofilms with C. albicans, with P. gingivalis exhibiting a 20% increase in viability under normoxic conditions in the presence of C. albicans [14].
One further key partnership that derives from the metabolism of dietary sugars and has strong links with the progression of early childhood caries is seen between C. albicans and S. mutans. As mentioned, these two microbes can form biofilm super-structures on the tooth surface that derive from their capacity to form complex aggregates. This is primarily driven by the synthesis of α-glucans from sucrose by glucosyltransferase B (GtfB), which is secreted by S. mutans and then bound to mannoproteins on the surface of C. albicans whilst remaining enzymatically active. The large quantities of α-glucan that are subsequently produced provide binding sites for S. mutans and effectively ‘glue’ the microbes together, with these aggregates then attaching more efficiently to the tooth surface [10, 55]. RNA-Seq analysis further highlights the importance of the metabolic interaction to this cross-kingdom partnership. In dual-species biofilms, the presence of C. albicans significantly modulates the expression of 393 S. mutans genes, the majority of which are associated with carbohydrate transport and metabolism [56]. For example, these dual-species biofilms exhibit upregulation of S. mutans genes associated with acid synthesis (ldh), aciduricity (fabM and atpD), acid-tolerance, bacterial adherence, and biofilm formation (e.g. ciaR and ciaH). C. albicans PHR2 is considered critical for this regulatory effect [8, 10, 56]. Again, maintenance of the highly acidic microenvironment because of this cross-kingdom interaction is what drives the progression of carious lesions.
Conclusion
Second only to the gut with regard to diversity, the oral microbiota comprises a complex polymicrobial community with members across several kingdoms. This inevitably leads to the development of a multitude of dynamic intermicrobial interactions, beneficial and antagonistic, as the microbial inhabitants strive to optimise their persistence within their specific oral niche(s). For C. albicans, the predominant fungus within the oral cavity, this has led to the evolution of specific partnerships with a range of oral bacteria, based on physical, chemical, and metabolic interactions. In many instances, these interactions facilitate C. albicans retention at the tooth or mucosal surface and through modulation of candidal morphogenesis, may even serve to maintain homeostasis within the community such that oral health is promoted. Often, however, synergistic effects promote microbial outgrowth or pathogenicity, generating a dysbiotic environment that exacerbates disease initiation and progression. Our understanding of the molecular mechanisms that underpin these cross-kingdom interactions continues to broaden. This new knowledge of oral ecology can be exploited to inform the design of interventions that can manipulate the microbiota for the benefit of oral health, and the development of novel therapeutics to combat oral disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bacali C, Vulturar R, Buduru S, Cozma A, Fodor A, Chiș A, et al. Oral microbiome: getting to know and befriend neighbors, a biological approach. Biomedicines. 2022;10(3): 671–92. https://doi.org/10.3390/biomedicines10030671. This review details the links between the oral microbiome and oral and systemic disease, and potential strategies for manipulating this polymicrobial community.
Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23(1):122–8. https://doi.org/10.4103/jomfp.JOMFP_304_18.
Bandara HM, Lam OL, Jin LJ, Samaranayake L. Microbial chemical signaling: a current perspective. Crit Rev Microbiol. 2012;38(3):217–49. https://doi.org/10.3109/1040841x.2011.652065.
Marsh PD, Zaura E. Dental biofilm: ecological interactions in health and disease. J Clin Periodontol. 2017;44(Suppl 18):S12-s22. https://doi.org/10.1111/jcpe.12679.
Georges FM, Do NT, Seleem D. Oral dysbiosis and systemic diseases. Frontiers in Dental Medicine. 2022;3:995423. https://doi.org/10.3389/fdmed.2022.995423. This review discusses the association of systemic diseases with changes in oral microbiome composition and thus oral disease risk.
Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6(1):e1000713. https://doi.org/10.1371/journal.ppat.1000713.
Patel M. Oral cavity and Candida albicans: colonisation to the development of infection. Pathogens. 2022;11(3):335–51. https://doi.org/10.3390/pathogens11030335.
Du Q, Ren B, He J, Peng X, Guo Q, Zheng L, et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J. 2021;15(3):894–908. https://doi.org/10.1038/s41396-020-00823-8.
Kim HE, Liu Y, Dhall A, Bawazir M, Koo H, Hwang G. Synergism of Streptococcus mutans and Candida albicans reinforces biofilm maturation and acidogenicity in saliva: an in vitro study. Front Cell Infect Microbiol. 2020;10:623980. https://doi.org/10.3389/fcimb.2020.623980.
Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82(5):1968–81. https://doi.org/10.1128/iai.00087-14.
Dige I, Nyvad B. Candida species in intact in vivo biofilm from carious lesions. Arch Oral Biol. 2019;101:142–6. https://doi.org/10.1016/j.archoralbio.2019.03.017.
Deng L, Li W, He Y, Wu J, Ren B, Zou L. Cross-kingdom interaction of Candida albicans and Actinomyces viscosus elevated cariogenic virulence. Arch Oral Biol. 2019;100:106–12. https://doi.org/10.1016/j.archoralbio.2019.02.008.
Shigeishi H, Nakamura M, Oka I, Su CY, Yano K, Ishikawa M, et al. The associations of periodontopathic bacteria and oral Candida with periodontal inflamed surface area in older adults receiving supportive periodontal therapy. Diagnostics 2021;11(8):1397–409. https://doi.org/10.3390/diagnostics11081397.
Bartnicka D, Karkowska-Kuleta J, Zawrotniak M, Satała D, Michalik K, Zielinska G, et al. Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment. Sci Rep. 2019;9(1):4376. https://doi.org/10.1038/s41598-019-40771-8.
Sztukowska MN, Dutton LC, Delaney C, Ramsdale M, Ramage G, Jenkinson HF, et al. Community development between Porphyromonas gingivalis and Candida albicans mediated by InlJ and Als3. mBio. 2018;9(2):e00202–18. https://doi.org/10.1128/mBio.00202-18.
Guo Y, Wang Y, Wang Y, Jin Y, Wang C. Heme competition triggers an increase in the pathogenic potential of Porphyromonas gingivalis in Porphyromonas gingivalis-Candida albicans mixed biofilm. Front Microbiol. 2020;11:596459. https://doi.org/10.3389/fmicb.2020.596459.
Bartnicka D, Gonzalez-Gonzalez M, Sykut J, Koziel J, Ciaston I, Adamowicz K, et al. Candida albicans shields the periodontal killer Porphyromonas gingivalis from recognition by the host immune system and supports the bacterial infection of gingival tissue. Int J Mol Sci. 2020;21(6). https://doi.org/10.3390/ijms21061984.
Oka I, Shigeishi H, Ohta K. Co-infection of oral Candida albicans and Porphyromonas gingivalis is associated with active periodontitis in middle-aged and older Japanese people. Medicina. 2022;58(6):723–32. https://doi.org/10.3390/medicina58060723.
Cavalcanti IM, Nobbs AH, Ricomini-Filho AP, Jenkinson HF, Del Bel Cury AA. Interkingdom cooperation between Candida albicans, Streptococcus oralis and Actinomyces oris modulates early biofilm development on denture material. Pathog Dis. 2016;74(3):ftw002. https://doi.org/10.1093/femspd/ftw002.
Fujinami W, Nishikawa K, Ozawa S, Hasegawa Y, Takebe J. Correlation between the relative abundance of oral bacteria and Candida albicans in denture and dental plaques. J Oral Biosci. 2021;63(2):175–83. https://doi.org/10.1016/j.job.2021.02.003.
Alnuaimi AD, Ramdzan AN, Wiesenfeld D, O’Brien-Simpson NM, Kolev SD, Reynolds EC, et al. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016;22(8):805–14. https://doi.org/10.1111/odi.12565.
Kaźmierczak-Siedlecka K, Dvořák A, Folwarski M, Daca A, Przewłócka K, Makarewicz W. Fungal gut microbiota dysbiosis and its role in colorectal, oral, and pancreatic carcinogenesis. Cancers. 2020;12(5):1326–38. https://doi.org/10.3390/cancers12051326.
Arzmi MH, Cirillo N, Lenzo JC, Catmull DV, O’Brien-Simpson N, Reynolds EC, et al. Monospecies and polymicrobial biofilms differentially regulate the phenotype of genotype-specific oral cancer cells. Carcinogenesis. 2019;40(1):184–93. https://doi.org/10.1093/carcin/bgy137.
Amaya Arbeláez MI, de Paula ESACA, Navegante G, Valente V, Barbugli PA, Vergani CE. Proto-oncogenes and cell cycle gene expression in normal and neoplastic oral epithelial cells stimulated with soluble factors from single and dual biofilms of Candida albicans and Staphylococcus aureus. Front Cell Infect Microbiol. 2021;11:627043. https://doi.org/10.3389/fcimb.2021.627043.
Bertolini M, Ranjan A, Thompson A, Diaz PI, Sobue T, Maas K, et al. Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 2019;15(4):e1007717. https://doi.org/10.1371/journal.ppat.1007717.
Vyhnalova T, Danek Z, Gachova D, Linhartova PB. The role of the oral microbiota in the etiopathogenesis of oral squamous cell carcinoma. Microorganisms. 2021;9(8):1549–71. https://doi.org/10.3390/microorganisms9081549. This review discusses current understanding of the association of the oral microbiota with oral squamous carcinoma.
Boisen G, Davies JR, Neilands J. Acid tolerance in early colonizers of oral biofilms. BMC Microbiol. 2021;21(1):45. https://doi.org/10.1186/s12866-021-02089-2.
Willaert RG. Adhesins of yeasts: protein structure and interactions. J Fungi. 2018;4(4):119–46. https://doi.org/10.3390/jof4040119.
Kim D, Koo H. Spatial design of polymicrobial oral biofilm in its native disease state. J Dent Res. 2020;99(6):597–603. https://doi.org/10.1177/0022034520909313. This paper uses high resolution microscopy to show that members of the dental plaque biofilm organise into defined, three-dimensionalstructures.
Nobbs AH, Jenkinson HF. Interkingdom networking within the oral microbiome. Microbes Infect. 2015;17(7):484–92. https://doi.org/10.1016/j.micinf.2015.03.008.
Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmür R, et al. Oral biofilm architecture on natural teeth. PLoS One. 2010;5(2):e9321. https://doi.org/10.1371/journal.pone.0009321.
Du Q, Ren B, Zhou X, Zhang L, Xu X. Cross-kingdom interaction between Candida albicans and oral bacteria. Front Microbiol. 2022;13:911623. https://doi.org/10.3389/fmicb.2022.911623.
Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME, Jenkinson HF. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect Immun. 2010;78(11):4644–52. https://doi.org/10.1128/iai.00685-10.
Koo H, Andes DR, Krysan DJ. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018;14(12):e1007342. https://doi.org/10.1371/journal.ppat.1007342.
Xu H, Sobue T, Bertolini M, Thompson A, Dongari-Bagtzoglou A. Streptococcus oralis and Candida albicans synergistically activate μ-calpain to degrade E-cadherin from oral epithelial junctions. J Infect Dis. 2016;214(6):925–34. https://doi.org/10.1093/infdis/jiw201.
Tamai R, Sugamata M, Kiyoura Y. Candida albicans enhances invasion of human gingival epithelial cells and gingival fibroblasts by Porphyromonas gingivalis. Microb Pathog. 2011;51(4):250–4. https://doi.org/10.1016/j.micpath.2011.06.009.
Siqueira JF Jr, Rôças IN. Diversity of endodontic microbiota revisited. J Dent Res. 2009;88(11):969–81. https://doi.org/10.1177/0022034509346549.
Du Q, Yuan S, Zhao S, Fu D, Chen Y, Zhou Y, et al. Coexistence of Candida albicans and Enterococcus faecalis increases biofilm virulence and periapical lesions in rats. Biofouling. 2021;37(9–10):964–74. https://doi.org/10.1080/08927014.2021.1993836.
Ren Z, Jeckel H, Simon-Soro A, Xiang Z, Liu Y, Cavalcanti IM, et al. Interkingdom assemblages in human saliva display group-level surface mobility and disease-promoting emergent functions. Proc Natl Acad Sci U S A. 2022;119(41):e2209699119. https://doi.org/10.1073/pnas.2209699119. This paper uses real-time microscopy and computational analysis to show that C. albicans and S. mutans form biofilm super-structures with enhanced mobility and pathogenicity.
Jack AA, Daniels DE, Jepson MA, Vickerman MM, Lamont RJ, Jenkinson HF, et al. Streptococcus gordonii comCDE (competence) operon modulates biofilm formation with Candida albicans. Microbiology. 2015;161:411–21. https://doi.org/10.1099/mic.0.000010.
Jarosz LM, Deng DM, van der Mei HC, Crielaard W, Krom BP. Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot Cell. 2009;8(11):1658–64. https://doi.org/10.1128/ec.00070-09.
Dixon EF, Hall RA. Noisy neighbourhoods: quorum sensing in fungal-polymicrobial infections. Cell Microbiol. 2015;17(10):1431–41. https://doi.org/10.1111/cmi.12490.
Cruz MR, Graham CE, Gagliano BC, Lorenz MC, Garsin DA. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect Immun. 2013;81(1):189–200. https://doi.org/10.1128/iai.00914-12.
Bamford CV, d’Mello A, Nobbs AH, Dutton LC, Vickerman MM, Jenkinson HF. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun. 2009;77(9):3696–704. https://doi.org/10.1128/iai.00438-09.
Polizzi A, Donzella M, Nicolosi G, Santonocito S, Pesce P, Isola G. Drugs for the quorum sensing inhibition of oral biofilm: new frontiers and insights in the treatment of periodontitis. Pharmaceutics. 2022;14(12):2740–57. https://doi.org/10.3390/pharmaceutics14122740.
Bachtiar EW, Bachtiar BM, Jarosz LM, Amir LR, Sunarto H, Ganin H, et al. AI-2 of Aggregatibacter actinomycetemcomitans inhibits Candida albicans biofilm formation. Front Cell Infect Microbiol. 2014;4:94. https://doi.org/10.3389/fcimb.2014.00094.
Vílchez R, Lemme A, Ballhausen B, Thiel V, Schulz S, Jansen R, et al. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). ChemBioChem. 2010;11(11):1552–62. https://doi.org/10.1002/cbic.201000086.
Kim D, Sengupta A, Niepa TH, Lee BH, Weljie A, Freitas-Blanco VS, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017;7:41332. https://doi.org/10.1038/srep41332.
Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52(5):782–9. https://doi.org/10.1093/jac/dkg449.
Miller DP, Fitzsimonds ZR, Lamont RJ. Metabolic signaling and spatial interactions in the oral polymicrobial community. J Dent Res. 2019;98(12):1308–14. https://doi.org/10.1177/0022034519866440.
Xu XL, Lee RT, Fang HM, Wang YM, Li R, Zou H, et al. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4(1):28–39. https://doi.org/10.1016/j.chom.2008.05.014.
Nasution O, Srinivasa K, Kim M, Kim YJ, Kim W, Jeong W, et al. Hydrogen peroxide induces hyphal differentiation in Candida albicans. Eukaryot Cell. 2008;7(11):2008–11. https://doi.org/10.1128/ec.00105-08.
Joyner PM, Liu J, Zhang Z, Merritt J, Qi F, Cichewicz RH. Mutanobactin A from the human oral pathogen Streptococcus mutans is a cross-kingdom regulator of the yeast-mycelium transition. Org Biomol Chem. 2010;8(24):5486–9. https://doi.org/10.1039/c0ob00579g.
Janus MM, Crielaard W, Volgenant CM, van der Veen MH, Brandt BW, Krom BP. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J Oral Microbiol. 2017;9(1):1270613. https://doi.org/10.1080/20002297.2016.1270613.
Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, Koo H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017;13(6):e1006407. https://doi.org/10.1371/journal.ppat.1006407.
He J, Kim D, Zhou X, Ahn SJ, Burne RA, Richards VP, et al. RNA-Seq reveals enhanced sugar metabolism in Streptococcus mutans co-cultured with Candida albicans within mixed-species biofilms. Front Microbiol. 2017;8:1036. https://doi.org/10.3389/fmicb.2017.01036.
Xu H, Sobue T, Bertolini M, Thompson A, Vickerman M, Nobile CJ, et al. S oralis activates the Efg1 filamentation pathway in C albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence. 2017;8(8):1602–17. https://doi.org/10.1080/21505594.2017.1326438.
Funding
GKW is supported by a University of Bristol Health Sciences PhD Studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wijesinghe, G.K., Nobbs, A.H. & Bandara, H.M.H.N. Cross-kingdom Microbial Interactions Within the Oral Cavity and Their Implications for Oral Disease. Curr Clin Micro Rpt 10, 29–35 (2023). https://doi.org/10.1007/s40588-023-00191-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-023-00191-9