Abstract
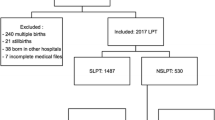
To estimate, for Lebanon, the financial benefit of screening for preterm pre-eclampsia (PE) at 11–13 weeks gestation combining risk factors with mean arterial pressure and maternal serum placental growth factor. Preterm PE cases delivered during 2010–2018 at Rafik Hariri University Hospital were identified from electronic records. Manual nursing notes were reviewed to confirm the diagnosis using international criteria. For each case, adverse maternal and infant events were noted and billing information extracted. A series of 1000 non-PE pregnancies were identified and billing information recorded. Published screening detection rates for a 10% false-positive rate and the proportion prevented by aspirin prophylaxis were applied to estimate the reduced cost following screening. There were a total of 17,131 deliveries including 486 (2.84%) PE and 223 (1.30%) preterm PE cases. The caesarean section rate was substantially higher for preterm PE (74%) than non-PE deliveries (36%) and 76% of infants were admitted to the Newborn Intensive Care Unit, where the average stay was 32, 21 and 8 days for deliveries before 32, 32–33 and 34–36 weeks respectively. The total cost of maternal and infant care for preterm PE was $881,206 and the average cost of an unaffected delivery $599. It was estimated that following screening the saving in treatment costs including aspirin would have been $431,665, which is $24 per woman delivering at the hospital over the nine year period. The financial savings are more than sufficient to pay for the screening test in those who are screen-positive.

Similar content being viewed by others
References
Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–20.
Ayala DE, Ucieda R, Hermida RC. Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int. 2013;30:260–79.
National Institute for Health and Clinical Excellence. Hypertension in pregnancy: the MANAGEMENT of hypertensive disorders during pregnancy. NICE clinical guidelines CG107;2010:1–47.
World Health Organisation. WHO recommendations for: Prevention and treatment of pre-eclampsia and eclampsia. Geneva; 2011.
American College of Obstetricians and Gynecologists. Committee Opinion number 743. Low-dose aspirin during pregnancy. ACOG 2018;132:e44–52.
O’Gorman N, Wright D, Poon LC, Rolnik D, Syngelaki A, de Alvarado M, et al. Multicenter screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: comparison to NICE guidelines and ACOG recommendations. Ultrasound Obstet Gynecol. 2017;49:756–60.
Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33(1):8–15 (Erratum in: Fetal Diagn Ther 2013;34(1):43).
Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018;51(6):743–50.
Park F, Russo K, Williams P, Pelosi M, Puddephatt R, Walter M, et al. Prediction and prevention of early-onset pre-eclampsia: impact of aspirin after first-trimester screening. Ultrasound Obstet Gynecol. 2015;46(4):419–23.
Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco MC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613–22.
Wright D, Poon LC, Rolnik DL, Syngelaki A, Delgado JL, Vojtassakova D, et al. Aspirin for evidence-based preeclampsia prevention trial: influence of compliance on beneficial effect of aspirin in prevention of preterm preeclampsia. Am J Obstet Gynecol. 2017;217(6):685.e1–5.
Sotiriadis A, Hernandez-Andrade E, da Silva Costa F, Ghi T, Glanc P, Khalil A, et al. ISUOG CSC Pre-eclampsia task force. ISUOG practice guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet Gynecol. 2019;53(1):7–22.
Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(Suppl 1):1–33.
Cuckle H, Neiger R. Prenatal screening strategies in localities with limited resources. J Fetal Med. 2017;4:165–70.
Lindheimer MD, Taler SJ, Cunningham FG. American Society of Hypertension position paper: hypertension in pregnancy. Clin Hypertens (Greenwich). 2009;11:214–25.
Sibai BM. Publications Committee, Society for Maternal-Fetal Medicine. Evaluation and management of severe pre-eclampsia. Am J Obstet Gynecol. 2011;205:191–8.
Lebanese ministry of public health. Drugs public price list according to the exchange rate issued on 21/12/2018.moph.gov.lb/en/DynamicPages/index/3/3101/drugs-public-price-list-#/en/view/20180/drugs-public-price-list-2018.
Donalson K, Turner S, Morrison L, Cuckle H. Maternal serum placental growth factor and α-fetoprotein testing in first trimester screening for Down’s syndrome. Prenat Diagn. 2013;33:1–5.
Johnson J, Pastuck M, Metcalf A, Nilsson C, Connors G, Krause R, et al. New approaches to first trimester Down’s syndrome screening using additional serum markers and cell free DNA. Prenat Diagn. 2013;33:1044–9.
Huang T, Dennis A, Meschino WS, Rashid S, Mak-Tam E, Cuckle H. First trimester screening for Down syndrome using nuchal translucency, maternal serum pregnancy-associated plasma protein A, free-β human chorionic gonadotrophin, placental growth factor and α-fetoprotein. Prenat Diagn. 2015;35(7):709–16.
Wright D, Syngelaki A, Bradbury I, Akolekar R, Nicolaides KH. First-trimester screening for trisomies 21, 18 and 13 by ultrasound and biochemical testing. Fetal Diagn Ther. 2014;35(2):118–26.
Carmichael JB, Liu HP, Janik D, Hallahan TW, Nicolaides KH, Krantz DA. Expanded conventional first trimester screening. Prenat Diagn. 2017;37(8):802–7.
Pourat N, Martinez AE, Jones, JM, Gregory KD, Korst L, Kominski GF. Costs of gestational hypertensive disorders in California: hypertension, preeclampsia, and eclampsia. Los Angeles (CA): UCLA Center for Health Policy Research; 2013:1–43.
Wright D, Rolnik DL, Syngelaki A, de Paco MC, Machuca M, de Alvarado M, et al. Aspirin for evidence-based preeclampsia prevention trial: effect of aspirin on length of stay in the neonatal intensive care unit. Am J Obstet Gynecol. 2018;218(6):612.e1–6.
Shmueli A, Meiri H, Gonen R. Economic assessment of screening for pre-eclampsia. Prenat Diagn. 2012;32(1):29–38.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HK is an employee of HVD Life Sciences GmbH and HC is a consultant to PerkinElmer Inc; both companies provide reagents for maternal serum preeclampsia tests. Other authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karaki, H., Khazaal, J., Chahine, R. et al. Cost-Effectiveness of First Trimester Screening for Preterm Pre-eclampsia in Lebanon. J. Fetal Med. 7, 119–123 (2020). https://doi.org/10.1007/s40556-019-00236-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40556-019-00236-4