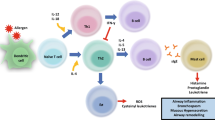
Abstract
Purpose of Review
This review analyzes the growing evidence regarding the standardized quality house dust mite (SQ-HDM) sublingual immunotherapy (SLIT) tablets as a valuable therapeutic option in asthma, as recently included in the treatment algorithm of the Global Initiative for Asthma (GINA) as an add-on treatment for adult patients with allergic asthma not well controlled with inhaled corticosteroids (ICS).
Recent Findings
Evidence from well-designed randomized placebo-controlled clinical trials shows that the SQ-HDM SLIT tablet is effective vs. placebo in reducing both asthma symptoms and the need for ICS, as well as the risk of experiencing a moderate/severe asthma exacerbation following ICS reduction, besides having a very good safety profile and tolerability. However, some unmet needs remain that need to be resolved.
Summary
The approval of the SQ-HDM SLIT tablet by regulatory authorities in Europe and the United States and its inclusion in the treatment recommendations in GINA open an opportunity window to enhance the range of management options in some patients with asthma by using an ethology-based treatment. Further studies are needed to facilitate the more complete evaluation of the therapeutic effect of this and other related products.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Eifan AO, Shamji MH, Durham SR. Long-term clinical and immunological effects of allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011;11:586–93.
Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013;132:1322–36.
• James C, Bernstein DI. Allergen immunotherapy: an updated review of safety. Curr Opin Allergy Clin Immunol. 2017;17(1):55–9 Comprehensive review of the most recent evidence on safety of AIT.
Allam J-P, Novak N. Immunological mechanisms of sublingual immunotherapy. Curr Opin Allergy Clin Immunol. 2014;14:564–9.
Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131:1288–96.
Nelson HS. Is sublingual immunotherapy ready for use in the United States? JAMA. 2013;309:1297–8.
Skoner D, Gentile D, Bush R, Fasano MB, McLaughlin A, Esch RE. Sublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollen. J Allergy Clin Immunol. 2010;125:660–6.
Bush RK, Swenson C, Fahlberg B, Evans MD, Esch R, Morris M, et al. House dust mite sublingual immunotherapy: results of a US trial. J Allergy Clin Immunol. 2011;127:974–81.
Wang L, Yin J, Fadel R, Montagut A, de Beaumont O, Devillier P. House dust mite sublingual immunotherapy is safe and appears to be effective in moderate, persistent asthma. Allergy. 2014;69(9):1181–8.
de Bot CM, Moed H, Berger MY, Röder E, Hop WC, de Groot H, et al. Sublingual immunotherapy not effective in house dust mite-allergic children in primary care. Pediatr Allergy Immunol. 2012;23(2):150–8.
ALK-Abelló A/S. Odactra. Highlights of prescribing information. 2017. Available from: https://www.odactra.com/assets/pdf/odactra-full-pi.pdf. Accessed 01 Jul 2018.
• Larenas Linnemann DES, Fernández Vega M, Luna Pech JA, Villaverde Rosas J, Ortega Martell JA, Del Río Navarro BE, et al. Pediatric asthma treatment: what to do when international guideline recommendations do not agree. Ann Allergy Asthma Immunol. 2018;121(1):7–13 This paper remarks differences between the recommendations on AIT for asthma treatment among different international clinical guidelines.
GINA Executive Committee. Global initiative for asthma; global strategy for asthma management and prevention. National Heart, Lung and Blood Institute, National Institute of Health: Bethesda (MD); 2015.
GINA Executive Committee. Global initiative for asthma; global strategy for asthma management and prevention. National Heart, Lung and Blood Institute, National Institute of Health: Bethesda (MD); 2017.
Oppenheimer J. The new mantra of asthma care-control. Ann Allergy Asthma Immunol. 2007;98(3):205–6.
Bateman ED, Reddel HK, Eriksson G, Peterson S, Ostlund O, Sears MR, et al. Overall asthma control: the relationship between current control and future risk. J Allergy Clin Immunol. 2010;125:600–8.
Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;8:CD001186.
• Asamoah F, Kakourou A, Dhami S, Lau S, Agache I, Muraro A, et al. Allergen immunotherapy for allergic asthma: a systematic overview of systematic reviews. Clin Transl Allergy. 2017;7:25 Systematic review focused on the evidence behind AIT as treatment for asthma.
•• Lin SY, Azar A, Suarez-Cuervo C, Diette GB, Brigham E, Rice J, et al. Role of sublingual immunotherapy in the treatment of asthma: an updated systematic review. Int Forum Allergy Rhinol. 2018;8(9):982–92 Systematic review stressing the current evidence on SLIT as treatment for asthma.
• Sánchez-Borges M, Fernandez-Caldas E, Thomas WR, Chapman MD, Lee BW, Caraballo L, et al. International consensus (ICON) on: clinical consequences of mite hypersensitivity, a global problem. World Allergy Organ J. 2017;10(1):14 Extensive review about the health impact of the sensitization to HDM.
Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol. 2015;136:38–48.
Platts-Mills TAE, Erwin EA, Heymann PW, Woodfolk JA. Pro: the evidence for a causal role of dust mites in asthma. Am J Respir Crit Care Med. 2009;180:109–21.
Tovey E, Ferro A. Time for new methods for avoidance of house dust mite and other allergens. Curr Allergy Asthma Rep. 2012;12(5):465–77.
Nurmatov U, Van Schayck CP, Hurwitz B, Sheikh A. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158–65.
Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. 2013;111(6):465–507.
ALK-Abelló A/S. Acarizax summary of product characteristics. 2015. Available from: http://mri.medagencies.org/download/DE_H_1947_001_FinalSPC.pdf. Accessed 05 Jul 2018.
• Henmar H, Frisenette SM, Grosch K, Nielsen K, Smith G, Sønderkær S, et al. Fractionation of source materials leads to a high reproducibility of the SQ house dust mite SLIT-tablets. Int Arch Allergy Immunol. 2016;169:23–32 This paper describes the standardization process for the SQ-HDM SLIT tablets.
•• Canonica GW, Virchow JC, Zieglmayer P, Ljørring C, Smith IM, Mosbech H. Efficacy and safety of SQ house dust mite (HDM) SLIT-tablet treatment of HDM allergic asthma. Expert Rev Clin Immunol. 2016;12(8):805–15 Comprehensive review assessing the complete drug profile of SQ-HDM SLIT tablets.
Corzo JL, Carrillo T, Pedemonte C, Plaza Martin AM, Martín Hurtado S, Dige E, et al. Tolerability during double-blind randomized phase I trials with the house dust mite allergy immunotherapy tablet in adults and children. J Investig Allergol Clin Immunol. 2014;24:154–61.
Penagos, M, Eifan, AO, Durham SR, Scadding GW. Duration of allergen immunotherapy for long-term efficacy in allergic rhinoconjunctivitis. Curr Treat Options Allergy. 2018;5:275–90.
• Nolte H, Bernstein DI, Kleine-Tebbe J, Fejerskov PA, Li Q, Lu S. Treatment with the SQ house dust mite sublingual immunotherapy tablet may be initiated year-round. J Allergy Clin Immunol Pract. 2018;6(5):1758–60 Clinical communication of 5 pooled RCTs regarding safety of year-round initiation of SQ-HDM SLIT tablets.
Frati F, Moingeon P, Marcucci F, Puccinelli P, Sensi L, Di Cara G, et al. Mucosal immunization application to allergic disease: sublingual immunotherapy. Allergy Asthma Proc. 2007;28(1):35–9.
Lawrence MG, Steinke JW, Borish L. Basic science for the clinician: mechanisms of sublingual and subcutaneous immunotherapy. Ann Allergy Asthma Immunol. 2016;117(2):138–42.
Jay DC, Nadeau KC. Immune mechanisms of sublingual immunotherapy. Curr Allergy Asthma Rep. 2014;14(11):473.
Mosbech H, Deckelmann R, De Blay F, Pastorello EA, Trebas-Pietras E, Andres LP, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568–75.
De Blay F, Kuna P, Prieto L, Ginko T, Seitzberg D, Riis B, et al. SQ-HDM SLIT-tablet (ALK) in treatment of asthma - post hoc results from a randomised trial. Respir Med. 2014;108:1430–7.
Nolte H, Maloney J, Nelson HS, Bernstein DI, Lu S, Li Z, et al. Onset and dose-related efficacy of house dust mite sublingual immunotherapy tablets in an environmental exposure chamber. J Allergy Clin Immunol. 2015;135:1494–501.
Nolte H, Maloney J, Nelson HS, Bernstein DI, Li Z, Jacobi H, et al. Effect of 12 SQ house dust mite sublingual immunotherapy tablet on asthma symptoms using an environmental exposure chamber. Ann Allergy Asthma Immunol. 2015;115(Suppl):A112.
• Virchow JC, Backer V, Kuna P, Prieto L, Nolte H, Villesen HH, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715–25 RCT evaluating the effect of HDM-SLIT tablets towards the risk to suffer asthma exacerbation after ICS withdrawal.
• Maloney J, Prenner BM, Bernstein DI, Lu S, Gawchik S, Berman G, et al. Safety of house dust mite sublingual immunotherapy standardized quality tablet in children allergic to house dust mites. Ann Allergy Asthma Immunol. 2016;116(1):59–65 Systematic review focused on the safety of HDM SLIT tablets in North America population.
Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization position paper 2009. World Allergy Organ J. 2009;2:233–81.
Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–6.
Comberiati P, Marseglia GL, Barberi S, Passalacqua G, Peroni DG. Allergen-specific immunotherapy for respiratory allergy in children: unmet needs and future goals. J Allergy Clin Immunol Pract. 2017;5(4):946–50.
ALK. Acarizax® approved in Europe for use in adolescent patients with house dust mite-induced allergic rhinitis. Available from: ir.alk.net/node/12891/pdf. Accessed 12 Oct 2018.
Marogna M, Spadolini I, Massolo A, Zanon P, Berra D, Chiodini E, et al. Effects of sublingual immunotherapy for multiple or single allergens in polysensitized patients. Ann Allergy Asthma Immunol. 2007;98(3):274–80.
Amar SM, Harbeck RJ, Sills M, Silveira LJ, O'Brien H, Nelson HS. Response to sublingual immunotherapy with grass pollen extract: monotherapy versus combination in a multiallergen extract. J Allergy Clin Immunol. 2009;124:150–6.
Bergmann KC, Demoly P, Worm M, Fokkens WJ, Carrillo T, Tabar AI, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–14.
Keles S, Karakos-Aydiner E, Ozen A, Izgi AG, Tevetoglu A, Akkoc T, et al. A novel approach in allergen-specific immunotherapy: combination of sublingual and subcutaneous routes. J Allergy Clin Immunol. 2011;128(4):808–15.
Ares Allergy Holdings PLC updates on the development of its house dust mite sublingual immunotherapy tablet in allergic asthma. Available from: http://stallergenesgreer.com/sites/default/files/Attachements/2015_10_23_stg320_aa_v072_pr_gb.pdf. Accessed 12 Oct 2018.
Passalacqua G. Recommendations for appropriate sublingual immunotherapy clinical trials. World Allergy Organ J. 2014;7(1):21.12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jorge A. Luna-Pech, MD, PhD, declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Allergic Asthma
Rights and permissions
About this article
Cite this article
Luna-Pech, J.A. House Dust Mite Tablets Now Officially Accepted as Treatment in GINA: What Is the Evidence and What’s Next?. Curr Treat Options Allergy 5, 424–435 (2018). https://doi.org/10.1007/s40521-018-0193-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-018-0193-1