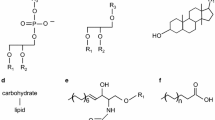
Abstract
Purpose of review
Herein, we summarize recent findings of novel lipid interactions between hosts and pathogens with the hope that further research is pursued in this emerging area of tropical disease lipidomics.
Recent findings
Hard-to-treat tropical diseases may be viral, parasitic, or bacterial in origin. Primarily caused in regions with high population densities, the lack of rapid diagnostic methods, effective long-term treatment regimens, and a high transmission rate results in major public health burdens. Intracellular niches occupied by the various pathogens in the human host further complicates treatment, as these pathogens present unique metabolic requirements utilizing lipids that are not shared by other extracellular pathogenic microorganisms. Improved techniques for comprehensive lipid analysis have renewed interest in exploring the mechanisms of these host-parasite lipid interactions with a focus on the host during infections. Maturation of mass spectrometry techniques coupled to lipidomic data processing has enabled pinpointing altered fatty acid compositions during infection, which are indicative of alterations to various lipid classes, and thus yield a common motif taken by pathogens to evade the host immune system and spread. This commonality includes various mechanisms that hijack host cell cholesterol metabolism and triacylglycerol biosynthesis.
Summary
Each pathogen modulates its own and hosts’ specific lipids as it occupies its host. Pathogens have distinctive interactions with the host lipidome for cell entry, scavenging for energy production, proliferation, and improved survival. These studies attempt to establish the foundational biochemical relationships that will either validate or suggest putative pathways, enhance diagnostics, evince novel therapeutic targets, and improve our overall understanding of these tropical diseases.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Röhrl C, Stangl H. Cholesterol metabolism—physiological regulation and pathophysiological deregulation by the endoplasmic reticulum. Wien Med Wochenschr. 2018;168:280–5.
Tidhar R, Futerman AH. The complexity of sphingolipid biosynthesis in the endoplasmic reticulum. Biochim Biophys Acta. 2013;1833:2511–8.
Gillespie LK, Hoenen A, Morgan G, Mackenzie JM. The endoplasmic reticulum provides the membrane platform for biogenesis of the Flavivirus replication complex. J Virol. 2010;84:10438–47.
Osuna-Ramos JF, Reyes-Ruiz JM, del Ángel RM. The role of host cholesterol during Flavivirus infection. Front Cell Infect Microbiol. 2018;8:388. https://doi.org/10.3389/fcimb.2018.00388.
Justis AV, Hansen B, Beare PA, King KB, Heinzen RA, Gilk SD. Interactions between the Coxiella burnetii parasitophorous vacuole and the endoplasmic reticulum involve the host protein ORP1L. Cell Microbiol. 2017. https://doi.org/10.1111/cmi.12637.
Goldston AM, Powell RR, Temesvari LA. Sink or swim: lipid rafts in parasite pathogenesis. Trends Parasitol. 2012;28:417–26.
Vallochi AL, Teixeira L, Oliveira KDS, Maya-Monteiro CM, Bozza PT. Lipid droplet, a key player in host-parasite interactions. Front Immunol. 2018;9:1022. https://doi.org/10.3389/fimmu.2018.01022.
Bozza PT, Bakker-Abreu I, Navarro-Xavier RA, Bandeira-Melo C. Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukot Essent Fattty Acids. 2011;85:205–13. https://doi.org/10.1016/j.plefa.2011.04.020.
Alberts, B. et al. Cell biology of infection. Mol. Biol. Cell 4th Ed. 2002.
Nagajyothi F, Weiss LM, Silver DL, et al. Trypanosoma cruzi utilizes the host low density lipoprotein receptor in invasion. PLoS Negl Trop Dis. 2011;5(2):e953. https://doi.org/10.1371/journal.pntd.0000953.
Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–66.
den Boon JA, Diaz A, Ahlquist P. Cytoplasmic viral replication complexes. Cell Host Microbe. 2010;8:77–85.
Heaton NS, Randall G. Dengue virus induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422–32.
•Marin-Palma D, Sirois CM, Urcuqui-Inchima S, Hernandez JC. Inflammatory status and severity of disease in dengue patients are associated with lipoprotein alterations. PLoS One. 2019;14:e0214245 Severe dengue heightens inflammation in their human host, as by increased levels of C reactive protein (CRP) among other cytokines. Activation of the inflammasome has been associated with reduced anti-inflammatory high-density lipoprotein (HDL); however, its relationship in Dengue infections with other lipids present in the host had not yet been explored. From plasma and serum, this study revealed a connection between the patient’s freely circulating cholesterol, HDL, and the inflammasome, suggesting that HDL could be used as a prospective biomarker for worsening infection.
El-Bacha T, et al. 1H nuclear magnetic resonance metabolomics of plasma unveils liver dysfunction in dengue patients. J Virol. 2016;90:7429–43.
Rothwell C, et al. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389:8–19.
Mackenzie JM, Khromykh AA, Parton RG. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe. 2007;2:229–39.
Soto-Acosta R, et al. The increase in cholesterol levels at early stages after dengue virus infection correlates with an augment in LDL particle uptake and HMG-CoA reductase activity. Virology. 2013;442:132–47.
Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antivir Res. 2013;98:192–208.
Jordan TX, Randall G. Flavivirus modulation of cellular metabolism. Curr Opin Virol. 2016;19:7–10.
Gutsche I, et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc Natl Acad Sci. 2011;108:8003–8.
Heaton NS, et al. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci U S A. 2010;107(17):345–17,350.
Aktepe TE, Pham H, Mackenzie JM. Differential utilisation of ceramide during replication of the flaviviruses West Nile and dengue virus. Virology. 2015;484:241–50.
Martín-Acebes MA, et al. The composition of West Nile virus lipid envelope unveils a role of sphingolipid metabolism in Flavivirus biogenesis. J Virol. 2014;88(12):041–12,054.
••Chotiwan N, et al. Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLoS Pathog. 2018;14:e1006853 Dengue, Zika, and other arboviruses are transmitted primarily by a mosquito vector, Aedes aegypti. Little had previously been known on the lipid profile of A. aegypti. Through lipid profiling and quantification via liquid chromatography mass spectrometry (LTQ Orbitrap XL), this study determined how dengue viruses impacted the lipidome of infected vector midguts, and most importantly revealed that the sphingolipid pathway was pertinent for dengue’s life cycle. Disruption of this pathway within the flaviviruses vector could effectively halt the transmission of these pathogens by mosquitos.
Voge NV, et al. Metabolomics-based discovery of small molecule biomarkers in serum associated with dengue virus infections and disease outcomes. PLoS Negl Trop Dis. 2016;10:e0004449.
•Villamor E, Villar LA, Lozano-Parra A, Herrera VM, Herrán OF. Serum fatty acids and progression from dengue fever to dengue haemorrhagic fever/dengue shock syndrome. Br J Nutr. 2018;120:787–96 As dengue fever (DF) infection progresses into dengue hemorrhagic fever (DHF) or “shock syndrome,” an emphasis by the community has been placed on uncovering roles lipids and their precursors may have on modulating these severe inflammatory responses. Polyunsaturated fatty acids (PUFA) dihomo-γ-linolenic acid (DHLA) and arachidonic acid (AA) were positively related to the worsening of DF. The results from this study can be leverage support for DHLA and AA as biomarkers for severe dengue fever.
Cui L, Lee YH, Kumar Y, et al. Serum metabolome and lipidome changes in adult patients with primary Dengue infection. PLoS Negl Trop Dis. 2013;7(8):e2373. https://doi.org/10.1371/journal.pntd.0002373.
•Queiroz A, Pinto IFD, Lima M, et al. Lipidomic Analysis Reveals Serum Alteration of Plasmalogens in Patients Infected With ZIKA Virus. Front Microbiol. 2019;10:753. https://doi.org/10.3389/fmicb.2019.00753. Zika virus (ZIKV) has been implicated in neurological complications and is a part of the flavivirus family. These arboviruses hijack host cell mechanisms such as membranous envelope biosynthesis and β-oxidation; however, little is known on what alterations within the serum occur to the host lipidome. This study is one of the forerunners to analyze the serum of ZIKV-infected individuals from a lipidomic perspective and characterized the biochemical pathways. This study revealed that PE-plasmalogens containing poly-unsaturated fatty acids increased by more than twofold, and that docosapentaenoic acid, dibomo-γ-linoleic acid, and arachidonic acid levels were elevated when compared with the control. Overall, this study indicates phospholipids as a potential biomarker for ZIKV.
Liang Q, et al. Zika virus NS4A and NS4B proteins deregulate Akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell. 2016;19:663–71.
Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41.
Han J, Wang Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell. 2018;9:145–51.
Stuart K, et al. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–10.
Filardy AA, Guimarães-Pinto K, Nunes MP, et al. Human Kinetoplastid Protozoan Infections: where Are We Going Next? Front Immunol. 2018;9:1493. https://doi.org/10.3389/fimmu.2018.01493.
Lantos AB, et al. Sialic acid glycobiology unveils Trypanosoma cruzi Trypomastigote membrane physiology. PLoS Pathog. 2016;12:e1005559.
Acosta-Serrano A., Mottram J. Comparison and evolution of the surface architecture of trypanosomatid parasites. In Trypanosomes: after the genome.
Paul KS, Jiang D, Morita YS, Englund PT. Fatty acid synthesis in African trypanosomes: a solution to the myristate mystery. Trends Parasitol. 2001;17:381–7.
Lee SH, Stephens JL, Paul KS, Englund PT. Fatty acid synthesis by elongases in trypanosomes. Cell. 2006;126:691–9.
Allmann S, Mazet M, Ziebart N, et al. Triacylglycerol Storage in Lipid Droplets in Procyclic Trypanosoma brucei. PLoS One. 2014;9(12). https://doi.org/10.1371/journal.pone.0114628.
•Sharma AI, et al. Sterol targeting drugs reveal life cycle stage-specific differences in trypanosome lipid rafts. Sci Rep. 2017;7:1–14 The singular cilia and flagella Trypanosoma brucei have are important in cell signaling and motion. Sterols are known to enrich these flagellar lipid rafts; however, the influence host lipids had on sterol incorporation during different stages of T. brucei’s lifecycle remained largely a mystery. By employing the drug treatments methyl-β-cyclodextrin (MBCD) and amphotericin B (Amp B), Sharma et al. were able to isolate the cholesterol species predominant in the flagellar lipid rafts for bloodstream form (BSF) T. brucei in mammalian hosts. Exploring this specific mechanism could result in the development of an agent capable of reducing cholesterol incorporation by T. brucei, disrupting the membrane mosaic of T. brucei, weakening extracellular signals.
Lamour SD, Gomez-Romero M, Vorkas PA, et al. Discovery of Infection Associated Metabolic Markers in Human African Trypanosomiasis. PLoS Negl Trop Dis. 2015;9(10):e0004200. https://doi.org/10.1371/journal.pntd.0004200.
Smith TK, Bütikofer P. Lipid metabolism in Trypanosoma brucei. Mol Biochem Parasitol. 2010;172:66–79.
Wang Y, et al. Global metabolic responses of mice to Trypanosoma brucei brucei infection. Proc Natl Acad Sci U S A. 2008;105:6127–32.
Chagas-Lima AC, et al. Bioactive lipids regulate Trypanosoma cruzi development. Parasitol Res. 2019;118:2609–19.
Prioli RP, Rosenberg I, Pereira ME. High- and low-density lipoproteins enhance infection of Trypanosoma cruzi in vitro. Mol Biochem Parasitol. 1990;38:191–8.
Johndrow C, Nelson R, Tanowitz H, Weiss L, Nagajyothi F. Trypanosoma cruzi infection results in an increase in intracellular cholesterol. Microbes Infect. 2014;16:337–44.
Nagajyothi F, et al. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol. 2012;14:634–43.
Ribeiro-Gomes FL., Lopes MF., DosReis GA Negative signaling and modulation of macrophage function in Trypanosoma cruzi infection. (Landes Bioscience, 2013).
•Lovo-Martins MI, Malvezi AD, Zanluqui NG, et al. Extracellular Vesicles Shed By T. cruzi Potentiate Infection and Elicit Lipid Body Formation and PGE2 Production in Murine Macrophages. Front Immunol. 2018;9:896. https://doi.org/10.3389/fimmu.2018.00896. During the initial stage of infection, Kinetoplastids must morphologically adapt to their new mammalian host and simultaneously evade a hostile host immune response. This article provides the putative mechanism by which Trypanosoma cruzi (strain Y) can avoid immune detection. Lovo-Martins et al. demonstrated that extracellular vesicles (EV) from T. cruzi increased prostaglandin E2 (PGE2) production while reducing pro-inflammatory cytokines (e.g., TNF-α and interleukin 6) via the controlled formation of lipid bodies (LB). Given this mechanism, further investigation into inhibiting the synthesis or altering the shedding of these vesicles would suggest an improved host response to infection.
Freire-de-Lima CG, et al. Proapoptotic activity of a Trypanosoma cruzi ceramide-containing glycolipid turned on in host macrophages by IFN-γ. J Immunol. 1998;161:4909–16.
Ramirez-Yañez GO, Hamlet S, Jonarta A, Seymour GJ, Symons AL. Prostaglandin E2 enhances transforming growth factor-beta 1 and TGF-beta receptors synthesis: an in vivo and in vitro study. Prostaglandins Leukot Essent Fat Acids. 2006;74:183–92.
Borges MM, et al. Prostaglandin and nitric oxide regulate TNF-α production during Trypanosoma cruzi infection. Immunol Lett. 1998;63:1–8.
••Colas RA, Ashton AW, Mukherjee S, et al. Trypanosoma cruzi produces the specialized proresolving mediators Resolvin D1, Resolvin D5, and Resolvin E2. Infect Immun. 2018;86(4):e00688–17. https://doi.org/10.1128/IAI.00688-17.Trypanosoma cruzi, the causative agent for Chagas disease, induce myocardial inflammation upon the invasion of host myoblasts. In response to inflammation, T. cruzi must evade recruited host immune cells and macrophages while meeting its energy needs to ensure survival. Fluctuating plasma levels of resolvins (Rv) D1, D5, and E2 along with the pro-inflammatory eicosanoid thromboxane A2 (TXA2) revealed that, in different life stages, T. cruzi synthesized these mediators, thus allowing the parasite to manipulate host’s inflammatory response. By isolating T. cruzi itself as the cause for stage-specific synthesis of vasoconstrictors and specialized proresolving mediators while in the host, investigators may now explore biochemical choke points that would disrupt these pathways, hindering T. cruzi’s capability to survive in the host.
Prevention, C.-C. for D. C. and. CDC - Leishmaniasis - epidemiology & risk factors. https://www.cdc.gov/parasites/leishmaniasis/epi.html. 2019. Accessed 19 Oct 2019.
•Semini G, Paape D, Paterou A, Schroeder J, Barrios-Llerena M, Aebischer T. Changes to cholesterol trafficking in macrophages by Leishmania parasites infection. Microbiologyopen. 2017. https://doi.org/10.1002/mbo3.469. While it has been known that the uptake of host’s cholesterol, as well as the endogenous biosynthesis of its own cholesterol, is modulated by Leishmania gene expression, little had been studied on how mechanistically cholesterol is trafficked and distributed throughout the parasite. In Semini et al., parasite-load impacted cholesterol sequestering, which occurred early in infection, acquiring one-third of its cholesterol from the host cell. A halo of exogenous cholesterol and fatty-acid bound low-density lipoprotein (LDL) formed around and inside of the parasitophourous vacuoles (PV) Leishmania utilizes to invade its host. Understanding how cholesterol is trafficked could lead to novel therapies that target this mechanism.
Roberts CW, et al. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol Biochem Parasitol. 2003;126:129–42.
Rub A, Arish M, Husain SA, Ahmed N, Akhter Y. Host-lipidome as a potential target of protozoan parasites. Microbes Infect. 2013;15:649–60.
Rabhi I, et al. Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view. PLoS Negl Trop Dis. 2012;6:e1763. https://doi.org/10.1371/journal.pntd.0001763.
Ali HZ, Harding CR, Denny PW. Endocytosis and Sphingolipid scavenging in Leishmania mexicana Amastigotes. Biochem Res Int. 2012. https://doi.org/10.1155/2012/691363.
Zufferey R, Al-Ani GK, Dunlap K. Leishmania dihydroxyacetonephosphate acyltransferase LmDAT is important for ether lipid biosynthesis but not for the integrity of detergent resistant membranes. Mol Biochem Parasitol. 2009;168:177–85.
Zhang O, Wilson MC, Xu W, et al. Degradation of host Sphingomyelin is essential for Leishmania virulence. PLoS Pathog. 2009;5(12):e1000692. https://doi.org/10.1371/journal.ppat.1000692
•Bouazizi-Ben Messaoud H, Guichard M, Lawton P, Delton I, Azzouz-Maache S. Changes in lipid and fatty acid composition during intramacrophagic transformation of Leishmania donovani Complex Promastigotes into Amastigotes. Lipids. 2017;52:433–41 Kinetoplastids such as Leishmania donovani must undergo major conformational changes throughout their life cycle, between their reproductive nonmotile amastigote stage and its parasitic promastigote stage. Understanding this shift in how different stages of L. donovani incorporate precursors or the parent lipid from the host, or how the parasite synthesizes these lipids, opens up the possibility for targeting these lipids in drug therapies as well as using them as biomarkers. Triacylglycerols (TAGs) and ergosterol were shown to decrease between infectious promastigote transforming to amastigote, while a phospholipid ratio shifted during the transition.
Biagiotti M, Dominguez S, Yamout N, Zufferey R. Lipidomics and anti-trypanosomatid chemotherapy. Clin Transl Med. 2017;6:27. https://doi.org/10.1186/s40169-017-0160-7.
Checchi F, et al. Malaria epidemics and interventions, Kenya, Burundi, Southern Sudan, and Ethiopia, 1999–2004. Emerg Infect Dis. 2006;12:1477–85.
Worrall E, Rietveld A, Delacollette C. The burden of malaria epidemics and cost-effectiveness of interventions in epidemic situations in Africa. Am J Trop Med Hyg. 2004;71:136–40.
van der Meer-Janssen YPM, van Galen J, Batenburg JJ, Helms JB. Lipids in host–pathogen interactions: Pathogens exploit the complexity of the host cell lipidome. Prog Lipid Res. 2010;49:1–26.
Hsiao LL, Howard RJ, Aikawa M, Taraschi TF. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite Plasmodium falciparum. Biochem J. 1991;274(Pt 1):121–32.
Simões AP, et al. Plasmodium knowlesi induces alterations in phosphatidylcholine and phosphatidylethanolamine molecular species composition of parasitized monkey erythrocytes. Biochim Biophys Acta. 1990;1022:135–45.
••Kluck GEG, et al. Plasmodium infection induces dyslipidemia and a hepatic lipogenic state in the host through the inhibition of the AMPK-ACC Pathway. Sci Rep. 2019;9:1–13 Plasmodium chadbaundi is a causative agent for malaria and relies on scavenging lipids from the host as it cannot synthesize certain lipid classes required for proliferation. Mouse plasma, when infected with P. chadbaundi, was studied and expressed AMP-activated protein kinase (AMPK) in hepatic cells. By blocking P. chadbaundi from altering the hepatic cell lipid metabolism, a potential drug therapy could be used to halt the proliferation and development of this parasite in vertebrate hosts.
Bhattacharjee S, Stahelin RV, Haldar K. Host targeting of virulence determinants and phosphoinositides in blood stage malaria parasites. Trends Parasitol. 2012;28:555–62.
Beaumelle BD, Vial HJ. Modification of the fatty acid composition of individual phospholipids and neutral lipids after infection of the simian erythrocyte by Plasmodium knowlesi. Biochim Biophys Acta B. 1986;877:262–70.
Wunderlich F, Fiebig S, Vial H, Kleinig H. Distinct lipid compositions of parasite and host cell plasma membranes from Plasmodium chabaudi-infected erythrocytes. Mol Biochem Parasitol. 1991;44:271–7.
Tran PN, et al. Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar J. 2016;15:73.
•Orikiiriza J, Surowiec I, Lindquist E, et al. Lipid response patterns in acute phase paediatric Plasmodium falciparum malaria. Metabolomics. 2017;13(4):41. https://doi.org/10.1007/s11306-017-1174-2. Literature concerning the characterization of the parasite Plasmodium falciparum during infection has largely been concerned with measuring lipoprotein density fractions or, when conducting further lipidomic analyses, have only studied in vitro or animal models. Little had been done in regard to understanding the lipidome of human subjects infected with malaria prior to this study. Untargeted and targeted liquid chromatography mass spectroscopy-quadrupole time of flight (LC-MSQTOF) analyses elucidated an association between higher concentrations of shorter-chain, highly saturated triacylglycerides (TAG) and lower levels of invasive P. falciparum in the plasma of pediatric patients. Conversely, higher levels of lysophosphatidylcholines (LPCs) were found with higher levels of parasite. Thus, these classes of lipids could serve as putative biomarkers for parasite load.
Kim M-J, Kim M-K, Kang J-S. Involvement of lipid rafts in the budding-like exit of Orientia tsutsugamushi. Microb Pathog. 2013;63:37–43.
Botelho-Nevers E, Rolain JM, Espinosa L, Raoult D. Statins limit Rickettsia conorii infection in cells. Int J Antimicrob Agents. 2008;32:344–8.
Ogawa M, et al. The intracellular pathogen Orientia tsutsugamushi responsible for scrub typhus induces lipid droplet formation in mouse fibroblasts. Microbes Infect. 2014;16:962–6.
Silverman DJ, Santucci LA, Meyers N, Sekeyova Z. Penetration of host cells by Rickettsia rickettsii appears to be mediated by a phospholipase of rickettsial origin. Infect Immun. 1992;60:2733–40.
Winkler HH, Day L, Daugherty R. Analysis of hydrolytic products from choline-labeled host cell phospholipids during growth of Rickettsia prowazekii. Infect Immun. 1994;62:1457–9.
Osterloh A. Immune response against rickettsiae: lessons from murine infection models. Med Microbiol Immunol. 2017;206:403–17.
Rydkina E, Sahni A, Baggs RB, Silverman DJ, Sahni SK. Infection of human endothelial cells with spotted fever group Rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins. Infect Immun. 2006;74:5067–74.
Walker TS, Brown JS, Hoover CS, Morgan DA. Endothelial prostaglandin secretion: effects of typhus Rickettsiae. J Infect Dis. 1990;162:1136–44.
Walker TS, Hoover CS. Rickettsial effects on leukotriene and prostaglandin secretion by mouse polymorphonuclear leukocytes. Infect Immun. 1991;59:351–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Monther Alsultan declares that he has no conflict of interest. Joshua Morriss declares that he has no conflict of interest. Daniel Contaifer Jr. declares that he has no conflict of interest. Naren Gajenthra Kumar declares that he has no conflict of interest. Dayanjan S. Wijesinghe declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on New Technologies and Advances in Infections Prevention
Rights and permissions
About this article
Cite this article
Alsultan, M., Morriss, J., Contaifer, D. et al. Host Lipid Response in Tropical Diseases. Curr Treat Options Infect Dis 12, 243–257 (2020). https://doi.org/10.1007/s40506-020-00222-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-020-00222-9