Abstract
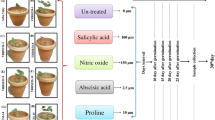
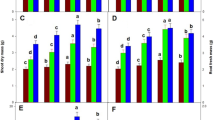
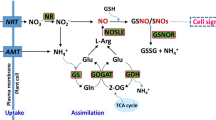
NADPH is one of the main reducing powers in plant cells. It plays important roles as cofactor or reductant and is involved in a wide range of anabolic pathways and thus may influence plant growth and development. The present study was conducted with the objective to analyse the effect of NADPH on plant growth and development when applied externally. External application of 10 µM and 30 µM NADPH resulted in 198% and 258%, increase in biomass of Arabidopsis thaliana plants, respectively. Under normal photoperiod of 16 h, 24% increase in seed germination was observed in 10 µM as well as 30 µM NADPH treated seeds on day 8 of the seed sowing. Further, targeted transcriptome profiling of all the eight genes encoding enzymes of oxidative pentose phosphate pathway revealed the differential expression of these genes under light and dark in response to NADPH. Interestingly, expression of glucose-6-phosphate-1-dehydrogenase and 6-phosphogluconolactonase, the first two enzymes of oxidative pentose phosphate pathway was up-regulated many fold under dark, whereas, NADPH treatment significantly increased the expression of the 2nd (6-phosphogluconolactonase) and 3rd gene (6-phosphogluconate dehydrogenase) of oxidative pentose phosphate pathway during light period. Proteomic analysis revealed thirty five proteins expressing differentially in response to NADPH treatment. These differentially expressed proteins were found to be involved in various biological processes. To the best of our knowledge this is the first report about the effect of external application of NADPH on plant biomass and seed germination. The information generated in the present study may be useful in having better insights into the role and influence of NADPH on plant growth and development and may provide platform to devise NADPH associated strategies for improving biomass production of useful plants.





Similar content being viewed by others
Abbreviations
- NADPH:
-
β-Nicotinamide adenine dinucleotide 2′-phosphate, reduced
- oxPPP:
-
Oxidative pentose phosphate pathway
- Glc-6-P:
-
Glucose-6-phosphate
- Ru-5-P:
-
Ribulose-5-phosphate
- Xul-5-P:
-
Xylulose-5-phosphate
- Rib-5-P:
-
Ribose-5-phosphate
- G6PDH:
-
Glucose-6-phosphate-1-dehydrogenase
- 6PGL:
-
6-Phosphogluconolactonase
- 6PGDH:
-
6-Phosphogluconate dehydrogenase
- RPI:
-
Ribose-5-phophate isomerase
- RPE:
-
Ribulose-5-phosphate-3-epimerase
- TK:
-
Transketolase
- TA:
-
Transaldolase
- G6PI:
-
Glucose-6-phosphate isomerase
References
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Calvin, M., & Benson, A. A. (1948). The path of carbon in photosynthesis. Science, 107, 476–480.
Dubouzet, J. G., Strabala, T. J., & Wagner, A. (2013). Potential transgenic routes to increase tree biomass. Plant Science, 212, 72–101.
Fan, J. X., Yang, X. X., Song, J. Z., Huang, X. M., Cheng, Z. X., Yao, L., et al. (2011). Heterologous expression of transaldolase gene Tal from Saccharomyces cerevisiae in Fusarium oxysporum for enhanced bioethanol production. Applied Biochemistry and Biotechnology, 164, 1023–1036.
Gangola, M. P., Parkash, J., Ahuja, P. S., & Dutt, S. (2013). Components of antioxidant system of Picrorhiza kurrooa exhibit different spatio-temporal behavior. Molecular Biology Reports, 40, 6593–6603.
Grave, K., Schawen, A. V., & Scheibe, R. (1994). Purification, characterization and cDNA sequence of glucose-6-phosphate dehydrogenase from potato (Solanurn tuberosum L.). The Plant Journal, 5, 353–361.
Jelakovic, S., Kopriva, S., Heinz, K., & Schulz, G. E. (2003). Structure and catalytic mechanism of the cytosolic d-ribulose-5-phosphate-3-epimerase from rice. Journal of Molecular Biology, 326, 127–135.
Kindl, H. (1993). Fatty acid degradation in plant peroxisome function and biosynthesis of enzyme involved. Biochimie, 75, 225–230.
Kruger, N. J., & Schawen, A. V. (2003). The oxidative pentose phosphate pathway; structure and organistaion. Current Opinion in Plant Biology, 6, 236–246.
Mekhalfi, M., Avilan, L., Lebrun, R., Botebol, H., & Gontero, B. (2012). Consequences of the presence of 24-epibrassinolide on cultures of a diatom Asterionella formosa. Biochimie, 94, 1213–1220.
Miclet, E., & Stoven, V. (2001). NMR spectroscopic analysis of the first two steps of the pentose-phosphate pathway elucidates the role of 6-phosphogluconolactonase. The Journal of biological chemistry, 276, 34840–34846.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco cultures. Physiologia Plantarum, 15, 473–497.
Parkash, J., Kashyap, S., Kalita, P. J., Devi, M., Ahuja, P. S., & Dutt, S. (2014). Differential proteomics of Picrorhiza kurrooa Royle ex Benth. in response to dark stress. Molecular Biology Reports, 41, 6051–6062.
Poirier, Y., Antonenkov, V. D., Glumoff, T., & Hiltunen, J. K. (2006). Peroxisomal beta-oxidation—A metabolic pathway with multiple functions. Biochimica et Biophysica Acta, 1763, 1413–1426.
Rajangam, A. S., Gidda, S. K., Craddock, C., Mullen, R. T., Dyer, J. M., & Eastmond, P. J. (2013). Molecular characterization of the fatty alcohol oxidation pathway for wax-ester mobilization in germinated Jojoba seeds. Plant Physiology, 161, 72–80.
Sanjeeta, K., Parkash, J., Kalita, P. J., Devi, M., Pathania, J., Joshi, R., et al. (2014). Comparative proteome analysis of Picrorhiza kurrooa Royle ex Benth. in response to drought. Journal of Proteome Science and Computational Biology, 3, 2.
Sharma, S. S., & Dietz, K. J. (2006). The significance of amino acids and amino acid derived molecule in plant responses and adaptation to heavy metal stress. Journal of Experimental Botany, 57, 711–726.
Voet, D., & Voet, J. G. (2011). Other pathways of carbohydrate metabolism. In Biochemistry (4th ed., pp. 871–892). Hoboken: Wiley.
Xiong, Y., DeFraia, C., Williams, D., Zhang, X., & Mou, Z. (2009a). Characterization of Arabidopsis 6-phosphogluconolactonase T-DNA insertion mutants reveals an essential role for the oxidative section of the plastidic pentose phosphate pathway in plant growth and development. Plant and Cell Physiology, 50, 1277–1291.
Xiong, Y., DeFraia, C., Williams, D., Zhang, X., & Mou, Z. (2009b). Deficiency in a cytosolic ribose-5-phosphate isomerase causes chloroplast dysfunction, late flowering and premature cell death in Arabidopsis. Physiologia Plantarum, 137, 249–263.
Zhang, R., Andersson, C. E., Savchenko, A., Skarina, T., Evdokimova, E., Beasley, S., et al. (2003). Structure of Escherichia coli ribose-5-phosphate isomerase: A ubiquitous enzyme of the pentose phosphate pathway and the Calvin cycle. Structure, 11, 31–42.
Acknowledgements
We greatly acknowledge Director, CSIR-IHBT, for providing overall guidance and infrastructural support to carry out the work. We thank Council of Scientific and Industrial Research (CSIR), India, for funding the project entitled “Increasing biomass potential of plants: exploring light-independent mechanisms: sanctioned vide sanction order number 41/3/EMPOWER/2012-PPD under EMPOWER scheme. The manuscript represents IHBT Publication No. 3604.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dutt, S., Kirti, S., Vaidya, T. et al. External application of NADPH enhances biomass accumulation, seed germination and modulates expression of oxidative pentose phosphate pathway genes in Arabidopsis. Ind J Plant Physiol. 23, 748–759 (2018). https://doi.org/10.1007/s40502-018-0420-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-018-0420-6