Abstract
Purpose of Review
This article provides a brief overview of cancer-preventive phytochemicals specifically targeting pancreatic cancer (PC) stem cells for prevention and treatment.
Recent Findings
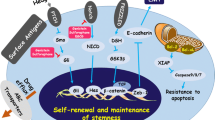
Cancer stem cells (CSCs) represent a small proportion of the total cells of a given tumor, and contribute to tumor growth, recurrence, metastasis, and treatment resistance. Many intertwined pathways, including hedgehog, Wnt Signaling, and NOTCH, have been shown to play a role in the formation of CSCs. Recently, numerous chemopreventive agents, such as genistein, (−)-epigallocatechin-3-gallate (EGCG), sulforaphane, curcumin, resveratrol, and quercetin, have been shown to target CSCs mediated through the inhibition of multiple signaling pathways, to avoid toxicity and the side effects of chemical compounds.
Summary
A growing body of research suggests that CSCs are the drivers in treatment resistance, cancer recurrence, and metastasis, in addition to tumor initiation and heterogeneity. Patient survival depends on these CSCs, which are one cause of tumor recurrence after surgery and chemotherapy. Therefore, target selection; an improved understanding of CSC biology, the genetic and molecular profiles of CSCs, and their key signaling pathways; and appropriate clinical trial endpoints that are designed to target CSCs will help in the development of drugs that will specifically target this small population of stem cells.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major Importance
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. (eds). SEER Cancer Statistics Review, 1975–2014. Bethesda: National Cancer Institute. https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
Rao CV, Mohammed A. New insights into pancreatic cancer stem cells. World J Stem Cells. 2015;7(3):547–55. https://doi.org/10.4252/wjsc.v7.i3.547.
Society’s AC. https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html.
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11. 2013. Lyon: International Agency for Research on Cancer; 2014.
Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102(12):2696–707. https://doi.org/10.1111/j.1572-0241.2007.01510.x.
Wittel UA, Momi N, Seifert G, Wiech T, Hopt UT, Batra SK. The pathobiological impact of cigarette smoke on pancreatic cancer development (review). Int J Oncol. 2012;41(1):5–14. https://doi.org/10.3892/ijo.2012.1414.
Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbeck's Arch Surg. 2008;393(4):535–45. https://doi.org/10.1007/s00423-007-0266-2.
Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, Swanson GM, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80(11):1830–7. https://doi.org/10.1038/sj.bjc.6690607.
Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer. 2007;120(9):1993–8. https://doi.org/10.1002/ijc.22535.
Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6(3):275–82. https://doi.org/10.1016/j.cgh.2007.12.041.
Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–83. https://doi.org/10.1038/sj.bjc.6602619.
Rao CV, Pal S, Mohammed A, Farooqui M, Doescher MP, Asch AS, et al. Biological effects and epidemiological consequences of arsenic exposure, and reagents that can ameliorate arsenic damage in vivo. Oncotarget. 2017;8(34):57605–21. https://doi.org/10.18632/oncotarget.17745.
•• Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol. 2015;21(11):3157–65. https://doi.org/10.3748/wjg.v21.i11.3157. Excellent review on update and comment on recent knowledge concerning PDAC biology and new diagnostics and treatment modalities of pancreatic cancer
Cascinu S, Falconi M, Valentini V, Jelic S, Group EGW. Pancreatic cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl_5):v55–v8.
Mohammed S, Van Buren G 2nd, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20(28):9354–60. https://doi.org/10.3748/wjg.v20.i28.9354.
Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91(21):9857–60.
Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, et al. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220(1):194–200. https://doi.org/10.1006/excr.1995.1306.
Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11842–9. https://doi.org/10.1073/pnas.2034201100.
Chang JC. Cancer stem cells: role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine (Baltimore). 2016;95(1 Suppl 1):S20–5. https://doi.org/10.1097/MD.0000000000004766.
Bao Q, Zhao Y, Renner A, Niess H, Seeliger H, Jauch KW, et al. Cancer stem cells in pancreatic cancer. Cancers (Basel). 2010;2(3):1629–41. https://doi.org/10.3390/cancers2031629.
•• Qiu H, Fang X, Luo Q, Ouyang G. Cancer stem cells: a potential target for cancer therapy. Cell Mol Life Sci. 2015;72(18):3411–24. https://doi.org/10.1007/s00018-015-1920-4. Excellent review on cancer stem cells in cancers as it presents the dysregulated properties and discusses the probable challenges in targeting CSCs for cancer treatment.
•• Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–7. https://doi.org/10.1158/0008-5472.CAN-06-2030. First original research article showing the presence of pancreatic stem cells and small population of cancer cells with high expression of cell surface markers (CD44, CD24, and epithelial-specific antigen [ESA]) in tumors.
Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, et al. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell. 2010;7(3):279–82. https://doi.org/10.1016/j.stem.2010.08.009.
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–23. https://doi.org/10.1016/j.stem.2007.06.002.
Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4(8):e6816. https://doi.org/10.1371/journal.pone.0006816.
Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141(6):2218–27 e5. https://doi.org/10.1053/j.gastro.2011.08.009.
Olempska M, Eisenach P, Ammerpohl O, Ungefroren H, Fandrich F, Kalthoff H. Detection of tumor stem cell markers in pancreatic carcinoma cell lines. Hepatobiliary Pancreat Dis Int. 2007;6(1):92–7.
Seguin L, Kato S, Franovic A, Camargo MF, Lesperance J, Elliott KC, et al. An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16(5):457–68. https://doi.org/10.1038/ncb2953.
Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102(5):340–51. https://doi.org/10.1093/jnci/djp535.
Adikrisna R, Tanaka S, Muramatsu S, Aihara A, Ban D, Ochiai T, et al. Identification of pancreatic cancer stem cells and selective toxicity of chemotherapeutic agents. Gastroenterology. 2012;143(1):234–245.e7. https://doi.org/10.1053/j.gastro.2012.03.054.
Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146(1):245–56. https://doi.org/10.1053/j.gastro.2013.09.050.
Wang YH, Li F, Luo B, Wang XH, Sun HC, Liu S, et al. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma. 2009;56(5):371–8.
•• Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97–106. https://doi.org/10.1038/nrclinonc.2010.196. Excellent review on targetiing cancer stem cells by inhibiting cross talk of Wnt, Notch, and Hedgehog pathways for the development of new anti-CSC therapeutic agents.
Merchant A, Matsui W. Targeting hedgehog—a cancer stem cell pathway. Clin Cancer Res. 2010;16(12):3130–40. https://doi.org/10.1158/1078-0432.CCR-09-2846.
Ng JMY, Curran T. The hedgehog’s tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11(7):493–501. https://doi.org/10.1038/nrc3079.
Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425(6960):846–51. https://doi.org/10.1038/nature01972.
Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104(14):5895–900. https://doi.org/10.1073/pnas.0700776104.
Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422(6929):313–7. https://doi.org/10.1038/nature01493.
Xia J, Chen C, Chen Z, Miele L, Sarkar FH, Wang Z. Targeting pancreatic cancer stem cells for cancer therapy. Biochim Biophys Acta. 2012;1826(2):385–99. https://doi.org/10.1016/j.bbcan.2012.06.002.
Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11(5):338–51. https://doi.org/10.1038/nrc3035.
Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400(6747):877–81. https://doi.org/10.1038/23716.
Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3(6):565–76.
Mazur PK, Einwächter H, Lee M, Sipos B, Nakhai H, Rad R, et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2010;107(30):13438–43. https://doi.org/10.1073/pnas.1002423107.
•• Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–73. https://doi.org/10.1038/onc.2016.304. Excellent review on Wnt signaling as it presents the current strategies to antagonize Wnt signaling in cancer and the challenges that are associated with such approaches.
McCleary-Wheeler AL, McWilliams R, Fernandez-Zapico ME. Aberrant signaling pathways in pancreatic cancer: a two compartment view. Mol Carcinog. 2012;51(1):25–39. https://doi.org/10.1002/mc.20827.
Morris JPT, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10(10):683–95. https://doi.org/10.1038/nrc2899.
Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. https://doi.org/10.1038/ncb2048.
Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, et al. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65(19):9064–72. https://doi.org/10.1158/0008-5472.CAN-05-1330.
Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307(1):26–36. https://doi.org/10.1016/j.canlet.2011.03.012.
Appari M, Babu KR, Kaczorowski A, Gross W, Herr I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int J Oncol. 2014;45(4):1391–400. https://doi.org/10.3892/ijo.2014.2539.
Kumazoe M, Takai M, Hiroi S, Takeuchi C, Yamanouchi M, Nojiri T, et al. PDE3 inhibitor and EGCG combination treatment suppress cancer stem cell properties in pancreatic ductal adenocarcinoma. Sci Rep. 2017;7(1):1917. https://doi.org/10.1038/s41598-017-02162-9.
Kallifatidis G, Rausch V, Baumann B, Apel A, Beckermann BM, Groth A, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58(7):949–63. https://doi.org/10.1136/gut.2008.149039.
Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, et al. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010;70(12):5004–13. https://doi.org/10.1158/0008-5472.CAN-10-0066.
Kallifatidis G, Labsch S, Rausch V, Mattern J, Gladkich J, Moldenhauer G, et al. Sulforaphane increases drug-mediated cytotoxicity toward cancer stem-like cells of pancreas and prostate. Mol Ther. 2011;19(1):188–95. https://doi.org/10.1038/mt.2010.216.
Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed). 2011;3:515–28.
Rodova M, Fu J, Watkins DN, Srivastava RK, Shankar S. Sonic hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal. PLoS One. 2012;7(9):e46083. https://doi.org/10.1371/journal.pone.0046083.
Li SH, Fu J, Watkins DN, Srivastava RK, Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol Cell Biochem. 2013;373(1–2):217–27. https://doi.org/10.1007/s11010-012-1493-6.
Kunnumakkara AB, Bordoloi D, Harsha C, Banik K, Gupta SC, Aggarwal BB. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin Sci. 2017;131(15):1781–99. https://doi.org/10.1042/CS20160935.
Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010;37(3):551–61.
Srivastava RK, Tang S-N, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed). 2011;3:515.
Nwaeburu CC, Bauer N, Zhao Z, Abukiwan A, Gladkich J, Benner A, et al. Up-regulation of microRNA let-7c by quercetin inhibits pancreatic cancer progression by activation of Numbl. Oncotarget. 2016;7(36):58367–80. https://doi.org/10.18632/oncotarget.11122.
Carlo-Stella C, Regazzi E, Garau D, Mangoni L, Rizzo MT, Bonati A, et al. Effect of the protein tyrosine kinase inhibitor genistein on normal and leukaemic haemopoietic progenitor cells. Br J Haematol. 1996;93(3):551–7.
Xu L, Xiang J, Shen J, Zou X, Zhai S, Yin Y, et al. Oncogenic MicroRNA-27a is a target for genistein in ovarian cancer cells. Anti Cancer Agents Med Chem. 2013;13(7):1126–32.
Pavese JM, Krishna SN, Bergan RC. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am J Clin Nutr. 2014;100(Suppl 1):431S–6S. https://doi.org/10.3945/ajcn.113.071290.
Xia J, Cheng L, Mei C, Ma J, Shi Y, Zeng F, et al. Genistein inhibits cell growth and invasion through regulation of miR-27a in pancreatic cancer cells. Curr Pharm Des. 2014;20(33):5348–53.
Kumar G, Pillare SP, Maru GB. Black tea polyphenols-mediated in vivo cellular responses during carcinogenesis. Mini-Rev Med Chem. 2010;10(6):492–505.
Maru GB, Kumar G, Ghantasala S, Tajpara P. Polyphenol(s)-mediated in vivo cellular responses during carcinogenesis. In: Watson RR, Preedy VR, Zibadi S, editors. Polyphenols in health and diseases. New York: Elsevier; 2013.
Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66(5):2500–5. https://doi.org/10.1158/0008-5472.CAN-05-3636.
Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2012;131(1):30–40.
Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11(3):495–510. https://doi.org/10.1208/s12248-009-9128-x.
Maru GB, Ramchandani AG, Kumar G, Garg R. Curcumin-mediated cellular responses in chemical carcinogenesis. In: Watson RR, Preedy VR, editors. Bioactive foods and extracts; 2010. p. 181–203.
Kumar G, Tajpara P, Bukhari AB, Ramchandani AG, De A, Maru GB. Dietary curcumin post-treatment enhances the disappearance of B (a) P-derived DNA adducts in mouse liver and lungs. Toxicol Rep. 2014;1:1181–94.
Kumar G, Tajpara P, Maru G. Dietary turmeric post-treatment decreases DMBA-induced hamster buccal pouch tumor growth by altering cell proliferation and apoptosis-related markers. J Environ Pathol Toxicol Oncol. 2012;31(4):295–312.
Yallapu MM, Ebeling MC, Khan S, Sundram V, Chauhan N, Gupta BK, et al. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol Cancer Ther. 2013;12(8):1471–80. https://doi.org/10.1158/1535-7163.MCT-12-1227.
Bisht S, Mizuma M, Feldmann G, Ottenhof NA, Hong SM, Pramanik D, et al. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol Cancer Ther. 2010;9(8):2255–64. https://doi.org/10.1158/1535-7163.MCT-10-0172.
Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72(1):335–45. https://doi.org/10.1158/0008-5472.CAN-11-2182.
Bao B, Ali S, Ahmad A, Azmi AS, Li Y, Banerjee S, et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One. 2012;7(12):e50165. https://doi.org/10.1371/journal.pone.0050165.
Bao B, Ali S, Kong D, Sarkar SH, Wang Z, Banerjee S, et al. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One. 2011;6(3):e17850. https://doi.org/10.1371/journal.pone.0017850.
Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, Aderka D, et al. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Investig. 2007;25(6):411–8.
Yoshida K, Toden S, Ravindranathan P, Han H, Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis. 2017;38(10):1036–46. https://doi.org/10.1093/carcin/bgx065.
Shankar S, Nall D, Tang SN, Meeker D, Passarini J, Sharma J, et al. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One. 2011;6(1):e16530. https://doi.org/10.1371/journal.pone.0016530.
Appari M, Babu KR, Kaczorowski A, Gross W, Herr I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int J Oncol. 2014;45(4):1391–400.
Cao C, Sun L, Mo W, Sun L, Luo J, Yang Z, et al. Quercetin mediates β-catenin in pancreatic cancer stem-like cells. Pancreas. 2015;44(8):1334–9.
Nwaeburu CC, Abukiwan A, Zhao Z, Herr I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol Cancer. 2017;16(1):23. https://doi.org/10.1186/s12943-017-0589-8.
Acknowledgements
This work is supported by grant from NIH/NCI RO1-CA213987. The authors like to thank Ms. Kathy Kyler and Ms. Priyanka Tilak for their assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Cancer Chemoprevention
Rights and permissions
About this article
Cite this article
Kumar, G., Farooqui, M. & Rao, C.V. Role of Dietary Cancer-Preventive Phytochemicals in Pancreatic Cancer Stem Cells. Curr Pharmacol Rep 4, 326–335 (2018). https://doi.org/10.1007/s40495-018-0145-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-018-0145-2