Abstract
Purpose of the Review
The purpose of this review is to summarize recent evidence supporting the use of reactive carbonyl species scavengers in the prevention and treatment of disease.
Recent Findings
The newly developed 2-aminomethylphenol class of scavengers shows great promise in preclinical trials for a number of diverse conditions including neurodegenerative diseases and cardiovascular disease. In addition, new studies with the thiol-based and imidazole-based scavengers have found new applications outside of adjunctive therapy for chemotherapeutics.
Summary
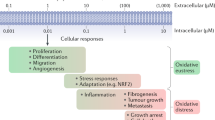
Reactive oxygen species (ROS) generated by cells and tissues not only act as signaling molecules and as cytotoxic agents to defend against pathogens but ROS also cause collateral damage to vital cellular components. The polyunsaturated fatty acyl chains of phospholipids in cell membranes are particularly vulnerable to damaging peroxidation by ROS. Evidence suggests that the breakdown of these peroxidized lipids to reactive carbonyls species plays a critical role in many chronic diseases. Antioxidants that abrogate ROS-induced formation of reactive carbonyl species also abrogate normal ROS signaling and thus exert both beneficial and adverse functional effects. The use of scavengers of reactive dicarbonyl species represents an alternative therapeutic strategy to potentially mitigate the adverse effects of ROS without abrogating normal signaling by ROS. In this review, we focus on three classes of reactive carbonyl species scavengers: thiol-based scavengers (2-mercaptoethanesulfonate and amifostine), imidazole-based scavengers (carnosine and its analogs), and 2-aminomethylphenol-based scavengers (pyridoxamine, 2-hydroxybenzylamine, and 5′-O-pentyl-pyridoxamine) that are either undergoing preclinical studies, advancing to clinical trials, or are already in clinical use.
Similar content being viewed by others
Abbreviations
- ACR:
-
Acrolein
- AngII:
-
Angiotensin II
- BSA:
-
Bovine serum albumin
- CEL:
-
N-carboxy-ethyl-lysine
- CML:
-
N-carboxy-methyl-lysine
- FDP:
-
Nɛ-(3-formyl-3,4-dehydropiperidino)
- HNE:
-
4-Hydroxynonenal
- 2-HOBA:
-
2-Hydroxybenzylamine or salicylamine
- HO•− :
-
Hydroxyl radical
- IsoLG:
-
Isolevuglandin
- MDA:
-
Malondialdehyde
- MESNA:
-
2-Mercaptoethanesulfonate
- MG-H1:
-
5-Methylimidazol-4-one
- MGO:
-
Methylglycoxal
- ONE:
-
4-Oxo-2-nonenal
- PE:
-
Phosphatidylethanolamine
- PM:
-
Pyridoxamine
- PPM:
-
5′-O-pentyl-pyridoxamine
- PUFA:
-
Polyunsaturated fatty acids
- RAGE:
-
Receptor for Advanced Glycation Endproducts
- STZ:
-
Streptozotocin
- TBARS:
-
Thiobarbituric acid reactive substances
- TNF:
-
Tumor necrosis factor
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, Cartwright K, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24(1):32–40.
Freedman BI, Wuerth J-P, Cartwright K, Bain RP, Dippe S, Hershon K, et al. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control Clin Trials. 1999;20(5):493–510. doi:10.1016/S0197-2456(99)00024-0.
Brown BE, Mahroof FM, Cook NL, van Reyk DM, Davies MJ. Hydrazine compounds inhibit glycation of low-density lipoproteins and prevent the in vitro formation of model foam cells from glycolaldehyde-modified low-density lipoproteins. Diabetologia. 2006;49(4):775–83. doi:10.1007/s00125-006-0137-3.
Liu W, Porter NA, Schneider C, Brash AR, Yin H. Formation of 4-hydroxynonenal from cardiolipin oxidation: intramolecular peroxyl radical addition and decomposition. Free Radic Biol Med. 2011;50(1):166–78. doi:10.1016/j.freeradbiomed.2010.10.709.
Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128.
Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992;89(10):4544–8.
Sayre LM, Arora PK, Iyer RS, Salomon RG. Pyrrole formation from 4-hydroxynonenal and primary amines. Chem Res Toxicol. 1993;6(1):19–22.
Brame CJ, Salomon RG, Morrow JD, Roberts 2nd LJ. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. 1999;274(19):13139–46.
Zhao J, Chen J, Zhu H, Xiong YL. Mass spectrometric evidence of malonaldehyde and 4-hydroxynonenal adductions to radical-scavenging soy peptides. J Agric Food Chem. 2012;60(38):9727–36. doi:10.1021/jf3026277.
Guichardant M, Taibi-Tronche P, Fay LB, Lagarde M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic Biol Med. 1998;25(9):1049–56.
Linhart KB, Glassen K, Peccerella T, Waldherr R, Linhart H, Bartsch H, et al. The generation of carcinogenic etheno-DNA adducts in the liver of patients with nonalcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2015;4(2):117–23. doi:10.3978/j.issn.2304-3881.2015.01.14.
Itakura K, Osawa T, Uchida K. Structure of a fluorescent compound formed from 4-hydroxy-2-nonenal and N(alpha)-hippuryllysine: a model for fluorophores derived from protein modifications by lipid peroxidation. J Org Chem. 1998;63(1):185–7.
Liu Y, Xu G, Sayre LM. Carnosine inhibits (E)-4-hydroxy-2-nonenal-induced protein cross-linking: structural characterization of Carnosine–HNE adducts. Chem Res Toxicol. 2003;16(12):1589–97. doi:10.1021/tx034160a.
Marchette LD, Wang H, Li F, Babizhayev MA, Kasus-Jacobi A. Carcinine has 4-hydroxynonenal scavenging property and neuroprotective effect in mouse retina. Invest Ophthalmol Vis Sci. 2012;53(7):3572–83. doi:10.1167/iovs.11-9042.
Menini S, Iacobini C, Ricci C, Scipioni A, Fantauzzi CB, Giaccari A, et al. D-carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br J Pharmacol. 2012;166(4):1344–56. doi:10.1111/j.1476-5381.2012.01834.x.
Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, et al. Pyridoxamine analogues scavenge lipid-derived gamma-ketoaldehydes and protect against H2O2-mediated cytotoxicity. Biochemistry. 2006;45(51):15756–67. doi:10.1021/bi061860g.
• Amarnath V, Amarnath K. Scavenging 4-oxo-2-nonenal. Chem Res Toxicol. 2015;28(10):1888–90. doi:10.1021/acs.chemrestox.5b00301. Describes novel pathway by which 2-aminomethylphenols react with ONE
• Vidal N, Cavaille JP, Graziani F, Robin M, Ouari O, Pietri S, et al. High throughput assay for evaluation of reactive carbonyl scavenging capacity. Redox Biol. 2014;2:590–8. doi:10.1016/j.redox.2014.01.016. Method of rapidly assessing lipid dicarbonyl reactivities and scavengers by use of fluorescent labeling
Zhang WH, Liu J, Xu G, Yuan Q, Sayre LM. Model studies on protein side chain modification by 4-oxo-2-nonenal. Chem Res Toxicol. 2003;16(4):512–23. doi:10.1021/tx020105a.
Galligan JJ, Rose KL, Beavers WN, Hill S, Tallman KA, Tansey WP, et al. Stable histone adduction by 4-oxo-2-nonenal: a potential link between oxidative stress and epigenetics. J Am Chem Soc. 2014;136(34):11864–6. doi:10.1021/ja503604t.
Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52(1):7–25. doi:10.1002/mnfr.200700412.
Yang Y, Yang Y, Trent MB, He N, Lick SD, Zimniak P, et al. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis. Atherosclerosis. 2004;173(2):211–21. doi:10.1016/j.atherosclerosis.2003.12.023.
Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57(1):6–15. doi:10.1093/toxsci/57.1.6.
Zemski Berry KA, Murphy RC. Characterization of acrolein-glycerophosphoethanolamine lipid adducts using electrospray mass spectrometry. Chem Res Toxicol. 2007;20(9):1342–51. doi:10.1021/tx700102n.
Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, et al. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A. 1998;95(9):4882–7.
McCall MR, Tang JY, Bielicki JK, Forte TM. Inhibition of lecithin-cholesterol acyltransferase and modification of HDL apolipoproteins by aldehydes. Arterioscler Thromb Vasc Biol. 1995;15(10):1599–606. doi:10.1161/01.atv.15.10.1599.
Zhu Q, Sun Z, Jiang Y, Chen F, Wang M. Acrolein scavengers: reactivity, mechanism and impact on health. Mol Nutr Food Res. 2011;55(9):1375–90. doi:10.1002/mnfr.201100149.
Bispo VS, de Arruda Campos IP, Di Mascio P, Medeiros MHG. Structural elucidation of a carnosine-acrolein adduct and its quantification in human urine samples. Scientific Reports. 2016;6:19348. doi:10.1038/srep19348. http://www.nature.com/articles/srep19348#supplementary-information
Salomon RG, Miller DB. Levuglandins: isolation, characterization, and total synthesis of new secoprostanoid products from prostaglandin endoperoxides. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:323–6.
Salomon RG, Jirousek MR, Ghosh S, Sharma RB. Prostaglandin endoperoxides 21. Covalent binding of levuglandin E2 with proteins. Prostaglandins. 1987;34(5):643–56.
Salomon RG, Subbanagounder G, Singh U, O’Neil J, Hoff HF. Oxidation of low-density lipoproteins produces levuglandin-protein adducts. Chem Res Toxicol. 1997;10(7):750–9. doi:10.1021/tx970016b.
Boutaud O, Brame CJ, Salomon RG, Roberts 2nd LJ, Oates JA. Characterization of the lysyl adducts formed from prostaglandin H2 via the levuglandin pathway. Biochemistry. 1999;38(29):9389–96. doi:10.1021/bi990470+.
Zagol-Ikapitte I, Bernoud-Hubac N, Amarnath V, Roberts 2nd LJ, Boutaud O, Oates JA. Characterization of bis(levuglandinyl) urea derivatives as products of the reaction between prostaglandin H2 and arginine. Biochemistry. 2004;43(18):5503–10. doi:10.1021/bi049842r.
Carrier EJ, Amarnath V, Oates JA, Boutaud O. Characterization of covalent adducts of nucleosides and DNA formed by reaction with levuglandin. Biochemistry. 2009;48(45):10775–81. doi:10.1021/bi9015132.
DiFranco E, Subbanagounder G, Kim S, Murthi K, Taneda S, Monnier VM, et al. Formation and stability of pyrrole adducts in the reaction of levuglandin E2 with proteins. Chem Res Toxicol. 1995;8(1):61–7.
• Bi W, Jang GF, Zhang L, Crabb JW, Laird J, Linetsky M, et al. Molecular structures of isolevuglandin-protein cross-links. Chem Res Toxicol. 2016;29(10):1628–40. doi:10.1021/acs.chemrestox.6b00141. Determination of the IsoLG crosslink structures and their dependence on protein sequence and other factors
Guo L, Chen Z, Cox BE, Amarnath V, Epand RF, Epand RM, et al. Phosphatidylethanolamines modified by gamma-ketoaldehyde (gammaKA) induce endoplasmic reticulum stress and endothelial activation. J Biol Chem. 2011;286(20):18170–80. doi:10.1074/jbc.M110.213470.
• Guo L, Chen Z, Amarnath V, Yancey PG, Van Lenten BJ, Savage JR, et al. Isolevuglandin-type lipid aldehydes induce the inflammatory response of macrophages by modifying phosphatidylethanolamines and activating the receptor for advanced glycation endproducts. Antioxid Redox Signal. 2015;22(18):1633–45. doi:10.1089/ars.2014.6078. Identifies IsoLG-PE as a mediator of macrophage activation via the RAGE receptor, and therefore a likely target of RCS scavenging
•• Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124(10):4642–56. doi:10.1172/JCI74084. Revealed a novel mechanisms by which IsoLG generation of neo-epitopes mediated hypertension and that 2-aminomethylphenols blocked development of hypertension
• Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126(4):1607. doi:10.1172/JCI87425. Pre-clinical study demonstrating that generation of IsoLG by NADPH oxidase contributes to aortic stiffening and hypertension and that 2-hydroxybenzylamine prevents this
Nakajima T, Davies SS, Matafonova E, Potet F, Amarnath V, Tallman KA, et al. Selective gamma-ketoaldehyde scavengers protect Nav1.5 from oxidant-induced inactivation. J Mol Cell Cardiol. 2010;48(2):352–9. doi:10.1016/j.yjmcc.2009.11.016.
Boyden PA, Davies SS, Viswanathan PC, Amarnath V, Balser JR, Roberts 2nd LJ. Potential role of isoketals formed via the isoprostane pathway of lipid peroxidation in ischemic arrhythmias. J Cardiovasc Pharmacol. 2007;50(5):480–6. doi:10.1097/FJC.0b013e31815a0564.
Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, et al. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97(12):1262–9. doi:10.1161/01.RES.0000195844.31466.e9.
• Sidorova TN, Yermalitskaya LV, Mace LC, Wells KS, Boutaud O, Prinsen JK, et al. Reactive gamma-ketoaldehydes promote protein misfolding and preamyloid oligomer formation in rapidly-activated atrial cells. J Mol Cell Cardiol. 2015;79:295–302. doi:10.1016/j.yjmcc.2014.11.013. Demonstration of 4-ketoaldehydes promoting the formation of preamyloid oligomers and the protective effects of 2-hydroxybenzylamine
Davies SS, Amarnath V, Montine KS, Bernoud-Hubac N, Boutaud O, Montine TJ, et al. Effects of reactive gamma-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 2002;16(7):715–7. doi:10.1096/fj.01-0696fje.
Foreman D, Levison BS, Miller DB, Salomon RG. Anhydrolevuglandin D2 inhibits the uterotonic activity of prostaglandins F2 alpha and D2. Prostaglandins. 1988;35(1):115–22.
Pryor WA, Stanley JP. Letter: a suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40(24):3615–7.
Chio KS, Tappel AL. Synthesis and characterization of the fluorescent products derived from malonaldehyde and amino acids. Biochemistry. 1969;8(7):2821–6.
Marnett LJ. Chemistry and biology of DNA damage by malondialdehyde. IARC Sci Publ. 1999;150:17–27.
Shuck SC, Wauchope OR, Rose KL, Kingsley PJ, Rouzer CA, Shell SM, et al. Protein modification by adenine propenal. Chem Res Toxicol. 2014;27(10):1732–42. doi:10.1021/tx500218g.
Zagol-Ikapite I, Sosa IR, Oram D, Judd A, Amarnath K, Amarnath V, et al. Modification of platelet proteins by malondialdehyde: prevention by dicarbonyl scavengers. J Lipid Res. 2015;56(11):2196–205. doi:10.1194/jlr.P063271.
Li X, Yang S, Lv X, Sun H, Weng J, Liang Y, et al. The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats. J Gynecol Oncol. 2013;24(2):177–85.
Kabasakal L, Şehirli AÖ, Çetinel Ş, Cikler E, Gedik N, Şener G. Mesna (2-mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sci. 2004;75(19):2329–40. doi:10.1016/j.lfs.2004.04.029.
Aydin AF, Kusku-Kiraz Z, Dogru-Abbasoglu S, Uysal M. Effect of carnosine treatment on oxidative stress in serum, apoB-containing lipoproteins fraction and erythrocytes of aged rats. Pharmacol Rep. 2010;62(4):733–9.
Nagasawa T, Yonekura T, Nishizawa N, Kitts DD. In vitro and in vivo inhibition of muscle lipid and protein oxidation by carnosine. Mol Cell Biochem. 2001;225(1):29–34.
• Rabbani N, Xue M, Thornalley PJ. Methylglyoxal-induced dicarbonyl stress in aging and disease: first steps towards glyoxalase 1-based treatments. Clin Sci. 2016;130(19):1677–96. doi:10.1042/cs20160025. Excellent review describing the formation, metabolism, and consequences of dicarbonyl stress in various diseases and emerging dicarbonyl-targeting therapeutics.
Allaman I, Bélanger M, Magistretti PJ. Methylglyoxal, the dark side of glycolysis. Front Neurosci. 2015;9:23. doi:10.3389/fnins.2015.00023.
Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem. 1994;269(51):32299–305.
Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324(Pt 2):565–70.
Brinkmann E, Wells-Knecht KJ, Thorpe SR, Baynes JW. Characterization of an imidazolium compound formed by reaction of methylglyoxal and N-alpha-hippuryllysine. J Chem Soc Perkin Trans. 1995;22:2817–8.
Rabbani N, Xue M, Thornalley PJ. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj J. 2016;33:513–25. doi:10.1007/s10719-016-9705-z.
Hipkiss AR, Chana H. Carnosine protects proteins against methylglyoxal-mediated modifications. Biochem Biophys Res Commun. 1998;248(1):28–32. doi:10.1006/bbrc.1998.8806.
Nagaraj RH, Sarkar P, Mally A, Biemel KM, Lederer MO, Padayatti PS. Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys. 2002;402(1):110–9. doi:10.1016/S0003-9861(02)00067-X.
Fuchs P, Loeseken C, Schubert JK, Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. Int J Cancer. 2010;126(11):2663–70. doi:10.1002/ijc.24970.
Alhamdani MSS, Al-Kassir AHAM, Jaleel NA, Hmood AM, Ali HM. Elevated levels of alkanals, alkenals and 4-HO-alkenals in plasma of hemodialysis patients. Am J Nephrol. 2006;26(3):299–303.
Corradi M, Pignatti P, Manini P, Andreoli R, Goldoni M, Poppa M, et al. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2004;24(6):1011–7. doi:10.1183/09031936.04.00002404.
Jalali M, Zare Sakhvidi MJ, Bahrami A, Berijani N, Mahjub H. Oxidative stress biomarkers in exhaled breath of workers exposed to crystalline silica dust by SPME-GC-MS. J Res Health Sci. 2016;16(3):153–61.
Zhou S, Decker EA. Ability of amino acids, dipeptides, polyamines, and sulfhydryls to quench hexanal, a saturated aldehydic lipid oxidation product. J Agric Food Chem. 1999;47(5):1932–6.
Townsend AJ, Leone-Kabler S, Haynes RL, Wu Y, Szweda L, Bunting KD. Selective protection by stably transfected human ALDH3A1 (but not human ALDH1A1) against toxicity of aliphatic aldehydes in V79 cells. Chem Biol Interact. 2001;130–132:261–73. doi:10.1016/S0009-2797(00)00270-2.
Tacka KA, Dabrowiak JC, Goodisman J, Souid A-K. Kinetic analysis of the reactions of 4-hydroperoxycyclophosphamide and acrolein with glutathione, mesna, and WR-1065. Drug Metab Dispos. 2002;30(8):875–82. doi:10.1124/dmd.30.8.875.
Brock N, Pohl J. The development of mesna for regional detoxification. Cancer Treat Rev. 1983;10(Suppl A):33–43.
Sakurai M, Saijo N, Shinkai T, Eguchi K, Sasaki Y, Tamura T, et al. The protective effect of 2-mercapto-ethane sulfonate (MESNA) on hemorrhagic cystitis induced by high-dose ifosfamide treatment tested by a randomized crossover trial. Jpn J Clin Oncol. 1986;16(2):153–6.
Fukuoka M, Negoro S, Masuda N, Furuse K, Kawahara M, Kodama N, et al. Placebo-controlled double-blind comparative study on the preventive efficacy of mesna against ifosfamide-induced urinary disorders. J Cancer Res Clin Oncol. 1991;117(5):473–8.
Vose JM, Reed EC, Pippert GC, Anderson JR, Bierman PJ, Kessinger A, et al. Mesna compared with continuous bladder irrigation as uroprotection during high-dose chemotherapy and transplantation: a randomized trial. J Clin Oncol. 1993;11(7):1306–10.
Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, et al. 2-mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-α release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011;50(11):1630–8. doi:10.1016/j.freeradbiomed.2011.03.009.
•• Hayslip J, Dressler EV, Weiss H, Taylor TJ, Chambers M, Noel T, et al. Plasma TNF-α and soluble TNF receptor levels after doxorubicin with or without co-administration of mesna—a randomized, cross-over clinical study. PLoS One. 2015;10(4):e0124988. doi:10.1371/journal.pone.0124988. First clinical demonstration of MESNA inhibiting inflammation when co-administered with redox-active chemotherapeutic doxorubicin.
Ludwig U, Riedel MK, Backes M, Imhof A, Muche R, Keller F. MESNA (sodium 2-mercaptoethanesulfonate) for prevention of contrast medium-induced nephrotoxicity - controlled trial. Clin Nephrol. 2011;75(4):302–8.
Amirshahrokhi K, Khalili A-R. Gastroprotective effect of 2-mercaptoethane sulfonate against acute gastric mucosal damage induced by ethanol. Int Immunopharmacol. 2016;34:183–8. doi:10.1016/j.intimp.2016.03.006.
Triantafyllidis I, Poutahidis T, Taitzoglou I, Kesisoglou I, Lazaridis C, Botsios D. Treatment with mesna and n-3 polyunsaturated fatty acids ameliorates experimental ulcerative colitis in rats. Int J Exp Pathol. 2015;96(6):433–43. doi:10.1111/iep.12163.
Arai T, Koyama R, Yuasa M, Kitamura D, Mizuta R. Acrolein, a highly toxic aldehyde generated under oxidative stress in vivo, aggravates the mouse liver damage after acetaminophen overdose. Biomed Res. 2014;35(6):389–95. doi:10.2220/biomedres.35.389.
Yilmaz ER, Kertmen H, Gürer B, Kanat MA, Arikok AT, Ergüder BI, et al. The protective effect of 2-mercaptoethane sulfonate (MESNA) against traumatic brain injury in rats. Acta Neurochir. 2013;155(1):141–9. doi:10.1007/s00701-012-1501-3.
Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-Spectrum Radioprotector. Oncologist. 2007;12(6):738–47. doi:10.1634/theoncologist.12-6-738.
Gu J, Zhu S, Li X, Wu H, Li Y, Hua F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta-analysis based on randomized controlled trials. PLoS One. 2014;9(5):e95968. doi:10.1371/journal.pone.0095968.
Kheradmand A, Nayebi AM, Jorjani M, Khalifeh S, Haddadi R. Effects of WR1065 on 6-hydroxydopamine-induced motor imbalance: possible involvement of oxidative stress and inflammatory cytokines. Neurosci Lett. 2016;627:7–12. doi:10.1016/j.neulet.2016.05.040.
Chronidou F, Apostolakis E, Papapostolou I, Grintzalis K, Georgiou CD, Koletsis EN, et al. Beneficial effect of the oxygen free radical scavenger amifostine (WR-2721) on spinal cord ischemia/reperfusion injury in rabbits. J Cardiothorac Surg. 2009;4(1):1–12. doi:10.1186/1749-8090-4-50.
Quinn PJ, Boldyrev AA, Formazuyk VE. Carnosine: its properties, functions and potential therapeutic applications. Mol Asp Med. 1992;13(5):379–444.
Aerts L, Van Assche FA. Low taurine, gamma-aminobutyric acid and carnosine levels in plasma of diabetic pregnant rats: consequences for the offspring. J Perinat Med. 2001;29(1):81–4. doi:10.1515/JPM.2001.012.
Gualano B, Everaert I, Stegen S, Artioli GG, Taes Y, Roschel H, et al. Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids. 2012;43(1):21–4. doi:10.1007/s00726-011-1165-y.
Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids. 2007;32(2):213–24. doi:10.1007/s00726-006-0409-8.
Harris RC, Wise JA, Price KA, Kim HJ, Kim CK, Sale C. Determinants of muscle carnosine content. Amino Acids. 2012;43(1):5–12. doi:10.1007/s00726-012-1233-y.
Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E, et al. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids. 2011;40(4):1221–9. doi:10.1007/s00726-010-0749-2.
Hipkiss AR. Carnosine and its possible roles in nutrition and health. Adv Food Nutr Res. 2009;57:87–154. doi:10.1016/S1043-4526(09)57003-9.
• Colzani M, De Maddis D, Casali G, Carini M, Vistoli G, Aldini G. Reactivity, selectivity, and reaction mechanisms of aminoguanidine, hydralazine, pyridoxamine, and carnosine as sequestering agents of reactive carbonyl species: a comparative study. ChemMedChem. 2016; doi:10.1002/cmdc.201500552. Systematic analyses of four carbonyl scavengers in their abilities to quench HNE, MDA, MGO and glyoxal which offers insight into improving scavenger design.
Orioli M, Aldini G, Benfatto MC, Facino RM, Carini M. HNE Michael adducts to histidine and histidine-containing peptides as biomarkers of lipid-derived carbonyl stress in urines: LC-MS/MS profiling in Zucker obese rats. Anal Chem. 2007;79(23):9174–84. doi:10.1021/ac7016184.
• Regazzoni L, de Courten B, Garzon D, Altomare A, Marinello C, Jakubova M, et al. A carnosine intervention study in overweight human volunteers: bioavailability and reactive carbonyl species sequestering effect. Sci Rep. 2016;6:27224. doi:10.1038/srep27224. Interventional study with carnosine that shows urinary excretion of carnosine-acrolein adducts depends on individual diversity of carnosine intake, metabolism, and basal RCS production.
Peters V, Schmitt CP, Zschocke J, Gross ML, Brismar K, Forsberg E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids. 2012;42(6):2411–6. doi:10.1007/s00726-011-1046-4.
Lee YT, Hsu CC, Lin MH, Liu KS, Yin MC. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol. 2005;513(1–2):145–50. doi:10.1016/j.ejphar.2005.02.010.
Peters V, Riedl E, Braunagel M, Hoger S, Hauske S, Pfister F, et al. Carnosine treatment in combination with ACE inhibition in diabetic rats. Regul Pept. 2014;194-195:36–40. doi:10.1016/j.regpep.2014.09.005.
Yan H, Guo Y, Zhang J, Ding Z, Ha W, Harding JJ. Effect of carnosine, aminoguanidine, and aspirin drops on the prevention of cataracts in diabetic rats. Mol Vis. 2008;14:2282–91.
Barski OA, Xie Z, Baba SP, Sithu SD, Agarwal A, Cai J, et al. Dietary carnosine prevents early atherosclerotic lesion formation in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2013;33(6):1162–70. doi:10.1161/ATVBAHA.112.300572.
• Menini S, Iacobini C, Ricci C, Blasetti Fantauzzi C, Pugliese G. Protection from diabetes-induced atherosclerosis and renal disease by D-carnosine-octylester: effects of early vs late inhibition of advanced glycation end-products in Apoe-null mice. Diabetologia. 2015;58(4):845–53. doi:10.1007/s00125-014-3467-6. Valuable pre-clinical study showing carnosine both inhibits early AGE formation and protects mice from diabetes-induced vascular and renal disease.
Menini S, Iacobini C, Ricci C, Scipioni A, Blasetti Fantauzzi C, Giaccari A, et al. D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br J Pharmacol. 2012;166(4):1344–56. doi:10.1111/j.1476-5381.2012.01834.x.
Min J, Senut MC, Rajanikant K, Greenberg E, Bandagi R, Zemke D, et al. Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J Neurosci Res. 2008;86(13):2984–91. doi:10.1002/jnr.21744.
Zhang X, Song L, Cheng X, Yang Y, Luan B, Jia L, et al. Carnosine pretreatment protects against hypoxia-ischemia brain damage in the neonatal rat model. Eur J Pharmacol. 2011;667(1–3):202–7. doi:10.1016/j.ejphar.2011.06.003.
Bae ON, Serfozo K, Baek SH, Lee KY, Dorrance A, Rumbeiha W, et al. Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke. 2013;44(1):205–12. doi:10.1161/STROKEAHA.112.673954.
• Davis CK, Laud PJ, Bahor Z, Rajanikant GK, Majid A. Systematic review and stratified meta-analysis of the efficacy of carnosine in animal models of ischemic stroke. J Cereb Blood Flow Metab. 2016; doi:10.1177/0271678X16658302. Systematic review of effects of carnosine administration in animal models of ischemic stroke.
Zhang ZY, Sun BL, Yang MF, Li DW, Fang J, Zhang S. Carnosine attenuates early brain injury through its antioxidative and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Cell Mol Neurobiol. 2015;35(2):147–57. doi:10.1007/s10571-014-0106-1.
Zieba R, Wagrowska-Danilewicz M. Influence of carnosine on the cardiotoxicity of doxorubicin in rabbits. Pol J Pharmacol. 2003;55(6):1079–87.
• de Courten B, Jakubova M, de Courten MP, Kukurova IJ, Vallova S, Krumpolec P, et al. Effects of carnosine supplementation on glucose metabolism: pilot clinical trial. Obesity. 2016;24(5):1027–34. doi:10.1002/oby.21434. Interventional pilot study showing that carnosine supplementation improved insulin sensitivity and glucose handling in obese and overweight individuals.
Lombardi C, Carubelli V, Lazzarini V, Vizzardi E, Bordonali T, Ciccarese C, et al. Effects of oral administration of orodispersible levo-carnosine on quality of life and exercise performance in patients with chronic heart failure. Nutrition. 2015;31(1):72–8. doi:10.1016/j.nut.2014.04.021.
Boldyrev A, Fedorova T, Stepanova M, Dobrotvorskaya I, Kozlova E, Boldanova N, et al. Carnosine [corrected] increases efficiency of DOPA therapy of Parkinson’s disease: a pilot study. Rejuvenation Res. 2008;11(4):821–7. doi:10.1089/rej.2008.0716.
Cornelli U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener Dis. 2010;7(1–3):193–202. doi:10.1159/000295663.
Babizhayev MA, Yermakova VN, Sakina NL, Evstigneeva RP, Rozhkova EA, Zheltukhina GA. N alpha-acetylcarnosine is a prodrug of L-carnosine in ophthalmic application as antioxidant. Clin Chim Acta. 1996;254(1):1–21.
Babizhayev MA, Micans P, Guiotto A, Kasus-Jacobi A. N-acetylcarnosine lubricant eyedrops possess all-in-one universal antioxidant protective effects of L-carnosine in aqueous and lipid membrane environments, aldehyde scavenging, and transglycation activities inherent to cataracts: a clinical study of the new vision-saving drug N-acetylcarnosine eyedrop therapy in a database population of over 50,500 patients. Am J Ther. 2009;16(6):517–33. doi:10.1097/MJT.0b013e318195e327.
Sakae K, Agata T, Kamide R, Yanagisawa H. Effects of L-carnosine and its zinc complex (Polaprezinc) on pressure ulcer healing. Nutr Clin Pract. 2013;28(5):609–16. doi:10.1177/0884533613493333.
Baraniuk JN, El-Amin S, Corey R, Rayhan R, Timbol C. Carnosine treatment for gulf war illness: a randomized controlled trial. Glob J Health Sci. 2013;5(3):69–81. doi:10.5539/gjhs.v5n3p69.
Sullivan D, Peterson R, Mujer C, Gad S. A 7-day intravenous toxicity study and neurotoxicity assessment of pyridorin in Sprague-Dawley rats. Human & Experimental Toxicology. 2016; doi:10.1177/0960327116661023.
Amarnath V, Amarnath K, Amarnath K, Davies S, Roberts 2nd LJ. Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem Res Toxicol. 2004;17(3):410–5.
Zagol-Ikapitte I, Amarnath V, Bala M, Roberts 2nd LJ, Oates JA, Boutaud O. Characterization of scavengers of gamma-ketoaldehydes that do not inhibit prostaglandin biosynthesis. Chem Res Toxicol. 2010;23(1):240–50.
Chipinda I, Mbiya W, Adigun RA, Morakinyo MK, Law BF, Simoyi RH, et al. Pyridoxylamine reactivity kinetics as an amine based nucleophile for screening electrophilic dermal sensitizers. Toxicology. 2014;315:102–9. doi:10.1016/j.tox.2013.11.009.
Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61(3):939–50. doi:10.1046/j.1523-1755.2002.00207.x.
Tanimoto M, Gohda T, Kaneko S, Hagiwara S, Murakoshi M, Aoki T, et al. Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-Ay/Ta mice. Metabolism. 2007;56(2):160–7. doi:10.1016/j.metabol.2006.08.026.
Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou M-M, et al. Role of transcription factor acetylation in diabetic kidney disease. Diabetes. 2014;63(7):2440–53. doi:10.2337/db13-1810.
• Maessen DE, Brouwers O, Gaens KH, Wouters K, Cleutjens JP, Janssen BJ, et al. Delayed intervention with pyridoxamine improves metabolic function and prevents adipose tissue inflammation and insulin resistance in high-fat diet-induced obese mice. Diabetes. 2016;65(4):956–66. doi:10.2337/db15-1390. Provocative pre-clinical study reporting that PM protects against high fat induced body weight gain.
• Skrypnyk NI, Voziyan P, Yang H, de Caestecker CR, Theberge M-C, Drouin M, et al. Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury. Am J Physiol Ren Physiol. 2016;311(2):F268–F77. doi:10.1152/ajprenal.00056.2016. First pre-clinical report of PM reducing short and long term injury and improving renal function after ischemia-reperfusion acute kidney injury.
Mastrocola R, Nigro D, Chiazza F, Medana C, Dal Bello F, Boccuzzi G, et al. Fructose-derived advanced glycation end-products drive lipogenesis and skeletal muscle reprogramming via SREBP-1c dysregulation in mice. Free Radic Biol Med. 2016;91:224–35. doi:10.1016/j.freeradbiomed.2015.12.022.
Watson AMD, Soro-Paavonen A, Sheehy K, Li J, Calkin AC, Koitka A, et al. Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia. 2011;54(3):681–9. doi:10.1007/s00125-010-2000-9.
Cao W, Chen J, Chen Y, Chen S, Chen X, Huang H, et al. Advanced glycation end products induced immune maturation of dendritic cells controls heart failure through NF-κB signaling pathway. Arch Biochem Biophys. 2015;580:112–20. doi:10.1016/j.abb.2015.07.003.
• Brodeur MR, Bouvet C, Bouchard S, Moreau S, Leblond J, de Blois D, et al. Reduction of advanced-glycation end products levels and inhibition of RAGE signaling decreases rat vascular calcification induced by diabetes. PLoS One. 2014;9(1):e85922. doi:10.1371/journal.pone.0085922. Pre-clinical study with PM showing efficacy to reduce diabetes-induced calcification in arteries in rats.
Wang C-H, Wu E-T, Wu M-S, Tsai M-S, Ko Y-H, Chang R-W, et al. Pyridoxamine protects against mechanical defects in cardiac ageing in rats: studies on load dependence of myocardial relaxation. Exp Physiol. 2014;99(11):1488–98. doi:10.1113/expphysiol.2014.082008.
Charvet CD, Saadane A, Wang M, Salomon RG, Brunengraber H, Turko IV, et al. Pretreatment with pyridoxamine mitigates isolevuglandin-associated retinal effects in mice exposed to bright light. J Biol Chem. 2013;288(41):29267–80. doi:10.1074/jbc.M113.498832.
Dong Z, Iwata D, Kitaichi N, Takeuchi M, Sato M, Endo N, et al. Amelioration of experimental autoimmune uveoretinitis by inhibition of glyceraldehyde-derived advanced glycation end-product formation. J Leukoc Biol. 2014;96(6):1077–85. doi:10.1189/jlb.3A0513-288RRR.
Thotala D, Chetyrkin S, Hudson B, Hallahan D, Voziyan P, Yazlovitskaya E. Pyridoxamine protects intestinal epithelium from ionizing radiation-induced apoptosis. Free Radic Biol Med. 2009;47(6):779–85. doi:10.1016/j.freeradbiomed.2009.06.020.
Yazlovitskaya EM, Voziyan PA, Manavalan T, Yarbrough WG, Ivanova AV. Cellular oxidative stress response mediates radiosensitivity in Fus1-deficient mice. Cell Death Dis. 2015;6:e1652. doi:10.1038/cddis.2014.593.
Williams ME, Bolton WK, Khalifah RG, Degenhardt TP, Schotzinger RJ, McGill JB. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am J Nephrol. 2007;27(6):605–14.
Lewis EJ, Greene T, Spitalewiz S, Blumenthal S, Berl T, Hunsicker LG, et al. Pyridorin in type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23(1):131–6. doi:10.1681/asn.2011030272.
Zagol-Ikapitte I, Matafonova E, Amarnath V, Bodine CL, Boutaud O, Tirona RG, et al. Determination of the pharmacokinetics and oral bioavailability of salicylamine, a potent γ-ketoaldehyde scavenger, by LC/MS/MS. Pharmaceutics. 2010;2(1):18–29. doi:10.3390/pharmaceutics2010018.
Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, et al. Treatment with a gamma-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. J Alzheimers Dis. 2011;27(1):49–59. doi:10.3233/JAD-2011-102118.
• Pearson JN, Warren E, Liang L-P, Roberts Ii LJ, Patel M. Scavenging of highly reactive gamma-ketoaldehydes attenuates cognitive dysfunction associated with epileptogenesis. Neurobiol Dis. 2017;98:88–99. doi:10.1016/j.nbd.2016.11.011. Preclinical study showing 2-hydroxybenzylamine reduces IsoLG adducts and blocks seizure-associated memory impairment.
• Nguyen TT, Caito SW, Zackert WE, West JD, Zhu S, Aschner M, et al. Scavengers of reactive gamma-ketoaldehydes extend Caenorhabditis elegans lifespan and healthspan through protein-level interactions with SIR-2.1 and ETS-7. Aging (Albany NY). 2016;8(8):1759–80. doi:10.18632/aging.101011. Provocative study showing 2-hydroxybenzylamine prolongs lifespans of C. elegans.
Lambert C, Li J, Jonscher K, Yang TC, Reigan P, Quintana M, et al. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J Biol Chem. 2007;282(27):19666–75. doi:10.1074/jbc.M611527200.
Oe T, Lee SH, Silva Elipe MV, Arison BH, Blair IA. A novel lipid hydroperoxide-derived modification to arginine. Chem Res Toxicol. 2003;16(12):1598–605. doi:10.1021/tx034178l.
Lee SH, Rindgen D, Bible Jr RH, Hajdu E, Blair IA. Characterization of 2′-deoxyadenosine adducts derived from 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chem Res Toxicol. 2000;13(7):565–74.
Rindgen D, Nakajima M, Wehrli S, Xu K, Blair IA. Covalent modifications to 2′-deoxyguanosine by 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chem Res Toxicol. 1999;12(12):1195–204.
Brinkmann E, Wells-Knecht KJ, Thorpe SR, Baynes JW. Characterization of an imidazolium compound formed by reaction of methylglyoxal and Nα-hippuryllysine. J Chem Soc Perkin Trans. 1995;1:2. doi:10.1039/P19950002817.
Frischmann M, Bidmon C, Angerer J, Pischetsrieder M. Identification of DNA adducts of methylglyoxal. Chem Res Toxicol. 2005;18(10):1586–92. doi:10.1021/tx0501278.
Acknowledgements
We thank Dr. Venkataraman Amarnath for his review of the manuscript and helpful suggestions. Dr. Davies and Zhang received funding from the National Heart, Lung, and Blood Institute grant HL116263-01A1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Davies is co-inventor on U.S. patent #7705054 for use of 2-aminomethylphenols as isolevuglandin scavengers for disorders involving oxidative injury.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human and animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines.
Additional information
This article is part of the Topical Collection on Chemical and Molecular Toxicology
Rights and permissions
About this article
Cite this article
Davies, S.S., Zhang, L.S. Reactive Carbonyl Species Scavengers—Novel Therapeutic Approaches for Chronic Diseases. Curr Pharmacol Rep 3, 51–67 (2017). https://doi.org/10.1007/s40495-017-0081-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-017-0081-6