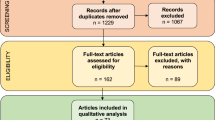
Abstract
Purpose of Review
This review provides an overview of the neurobiological mechanisms underlying opioid use disorder (OUD) drawing from genetic, functional, and structural magnetic resonance imaging (MRI) research.
Recent Findings
Preliminary evidence suggests an association between OUD and specific variants of the DRD2, δ-opioid receptor 1 (OPRD1), and μ-opioid receptor 1 (OPRM1) genes. Additionally, MRI research indicates functional and structural alterations in striatal and corticolimbic brain regions and pathways underlying reward, emotion/stress, and cognitive control processes among individuals with OUD.
Summary
Individual differences in genetic and functional and structural brain-based features are correlated with differences in OUD severity and treatment outcomes, and therefore may potentially one day be used to inform OUD treatment selection. However, given the heterogeneous findings reported, further longitudinal research across different stages of opioid addiction is needed to yield a convergent characterization of OUD and improve treatment and prevention.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001-2014. JAMA Pediatr. 2017;171(8):747–55.
O'Donnell JK, Gladden RM, Seth P. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region - United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(34):897–903.
Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357–68.
Bart G. Maintenance medication for opiate addiction: the Foundation of Recovery. J Addict Dis. 2012;31(3):207–25.
Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. A systematic review on the use of psychosocial interventions in conjunction with medications for the treatment of opioid addiction. J Addict Med. 2016;10(2):91–101.
Kouimtsidis C, Reynolds M, Coulton S, Drummond C. How does cognitive behaviour therapy work with opioid-dependent clients? Results of the UKCBTMM study. Drugs: Educ Prev Polic. 2012;19(3):253–8.
Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Nuro KF, et al. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiatry. 2008;165(7):881–8.
Hser YI, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Zhu Y, et al. High mortality among patients with opioid use disorder in a large healthcare system. J Addict Med. 2017;11(4):315–9.
Hendershot CS, Witkiewitz K, George WH, Marlatt GA. Relapse prevention for addictive behaviors. Subst Abuse Treat Prev Policy. 2011;6:17.
Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–46.
Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. JAMA. 2008;300(17):2003–11.
Bertschy G. Methadone maintenance treatment: an update. Eur Arch Psychiatry Clin Neurosci. 1995;245(2):114–24.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38.
Abi-Dargham A, Horga G. The search for imaging biomarkers in psychiatric disorders. Nat Med. 2016;22(11):1248–55.
•• Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19(8):499–514 This review discusses the role of the opioid receptors in addiction and the translational potential of genetic, pharmacological and neuroimaging research in OUD.
Pert CB, Snyder SH. Properties of opiate-receptor binding in rat brain. Proc Natl Acad Sci. 1973;70(8):2243–7.
Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267(5611):495–9.
Benarroch EE. Endogenous opioid systems: current concepts and clinical correlations. Neurology. 2012;79(8):807–14.
Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115(6):1363–81.
Toll L, Bruchas MR, Cox BM, Zaveri NT. Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev. 2016;68(2):419–57.
Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700(1–2):89–98.
Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012;124(3):223–8.
Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, et al. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45(2):330–4.
Fields HL, Margolis EB. Understanding Opioid Reward. Trends Neurosci. 2015;38(4):217–25.
Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89(4):1379–412.
Richards EM, Mathews DC, Luckenbaugh DA, Ionescu DF, Machado-Vieira R, Niciu MJ, et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology. 2016;233(6):1119–30.
Ayanga D, Shorter D, Kosten TR. Update on pharmacotherapy for treatment of opioid use disorder. Expert Opin Pharmacother. 2016;17(17):2307–18.
Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63–75.
Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209.
Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207.
D'Onofrio G, O'Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–44.
• Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, et al. Extended-release injectable naltrexone for opioid use disorder: a systematic review. Addiction. 2018;113(7):1188–209 This systematic review examined extended-release injectable naltrexone (XR-NTX) for opioid use disorder, with regards to induction and adherence rates to XR-NTX as well as its effect on opioid use outcomes.
Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333.
Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–13.
• Tanum L, Solli KK, Latif ZE, Benth JS, Opheim A, Sharma-Haase K, et al. Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial. JAMA Psychiatry. 2017;74(12):1197–205 This randomized clinical trial demonstrates that injectable extended-release naltrexone is as effective as buprenorphine-naloxone in maintaining short-term abstinence from opioids.
Carroll KM, Nich C, Frankforter TL, Yip SW, Kiluk BD, DeVito EE, et al. Accounting for the uncounted: physical and affective distress in individuals dropping out of oral naltrexone treatment for opioid use disorder. Drug Alcohol Depend. 2018;192:264–70.
Samples H, Williams AR, Olfson M, Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of Medicaid enrollees. J Subst Abus Treat. 2018;95:9–17.
Berrettini W. A brief review of the genetics and pharmacogenetics of opioid use disorders. Dialogues Clin Neurosci. 2017;19(3):229–36.
Clarke TK, Weiss AR, Ferarro TN, Kampman KM, Dackis CA, Pettinati HM, et al. The dopamine receptor D2 (DRD2) SNP rs1076560 is associated with opioid addiction. Ann Hum Genet. 2014;78(1):33–9.
Bart G, Heilig M, LaForge K, Pollak L, Leal S, Ott J, et al. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in Central Sweden. Mol Psychiatry. 2004;9(6):547–9.
Drakenberg K, Nikoshkov A, Horváth MC, Fagergren P, Gharibyan A, Saarelainen K, et al. μ opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci. 2006;103(20):7883–8.
Coller JK, Beardsley J, Bignold J, Li Y, Merg F, Sullivan T, et al. Lack of association between the A118G polymorphism of the mu opioid receptor gene (OPRM1) and opioid dependence: a meta-analysis. Pharmacogenomics Pers Med. 2009;2:9–19.
Haerian BS, Haerian MS. OPRM1 rs1799971 polymorphism and opioid dependence: evidence from a meta-analysis. Pharmacogenomics. 2013;14(7):813–24.
Woodcock EA, Lundahl LH, Burmeister M, Greenwald MK. Functional mu opioid receptor polymorphism (OPRM1 A118G) associated with heroin use outcomes in Caucasian males: a pilot study. Am J Addict. 2015;24(4):329–35.
Crist RC, Clarke TK, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, et al. An intronic variant in OPRD1 predicts treatment outcome for opioid dependence in African-Americans. Neuropsychopharmacology. 2013;38(10):2003–10.
Clarke TK, Crist RC, Ang A, Ambrose-Lanci LM, Lohoff FW, Saxon AJ, et al. Genetic variation in OPRD1 and the response to treatment for opioid dependence with buprenorphine in European-American females. Pharmacogenomics J. 2014;14(3):303–8.
Crist RC, Doyle GA, Nelson EC, Degenhardt L, Martin NG, Montgomery GW, et al. A polymorphism in the OPRM1 3′-untranslated region is associated with methadone efficacy in treating opioid dependence. Pharmacogenomics J. 2016;18:173.
• Smith AH, Jensen KP, Li J, Nunez Y, Farrer LA, Hakonarson H, et al. Genome-wide association study of therapeutic opioid dosing identifies a novel locus upstream of OPRM1. Mol Psychiatry. 2017;22(3):346–52 This genome-wide association study identified a significant association between methadone dose and a single nucleotide polymorphism closely located to the OPRM1 gene in African Americans, but not European Americans.
Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254–62.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91.
Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700.
Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78(3):204–9.
Avvisati R, Contu L, Stendardo E, Michetti C, Montanari C, Scattoni ML, et al. Ultrasonic vocalization in rats self-administering heroin and cocaine in different settings: evidence of substance-specific interactions between drug and setting. Psychopharmacology. 2016;233(8):1501–11.
De Pirro S, Galati G, Pizzamiglio L, Badiani A. The affective and neural correlates of heroin vs. cocaine use in addiction are influenced by environmental setting but in opposite directions. Journal of Neuroscience. 2018;38(22):5182–95.
Hartwell KJ, Back SE, McRae-Clark AL, Shaftman SR, Brady KT. Motives for using: a comparison of prescription opioid, marijuana and cocaine dependent individuals. Addict Behav. 2012;37(4):373–8.
Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66(1):88–94.
• Ahn W-Y, Vassileva J. Machine-learning identifies substance-specific behavioral markers for opiate and stimulant dependence. Drug Alcohol Depend. 2016;161:247–57 This study used a machine-learning approach to identify multivariate substance-specific markers that classify heroin and amphetamine dependence respectively.
Ahn WY, Ramesh D, Moeller FG, Vassileva J. Utility of machine-learning approaches to identify behavioral markers for substance use disorders: impulsivity dimensions as predictors of current cocaine dependence. Front Psychiatry. 2016;7:34.
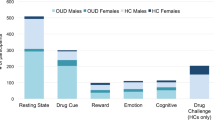
•• Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacology. 2018;44:259–73. This is a systematic review of fMRI studies in opioid use disorder, including studies comparing opioid-dependent and healthy control participants as well as studies on opioid medications, treatment and abstinence effects.
Li Q, Li W, Wang H, Wang Y, Zhang Y, Zhu J, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: an event-related functional magnetic resonance imaging study. Addict Biol. 2015;20(5):968–78.
Li Q, Yang WC, Wang YR, Huang YF, Li W, Zhu J, et al. Abnormal function of the posterior cingulate cortex in heroin addicted users during resting-state and drug-cue stimulation task. Chin Med J. 2013;126(4):734–9.
Lou M, Wang E, Shen Y, Wang J. Cue-elicited craving in heroin addicts at different abstinent time: an fMRI pilot study. Subst Use Misuse. 2012;47(6):631–9.
•• Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374(4):363–71 This article briefly summarizes current knowledge on neuroscience addiction research, including conceptual frameworks and neural circuitry underlying addiction.
Wang ZX, Zhang JX, Wu QL, Liu N, Hu XP, Chan RC, et al. Alterations in the processing of non-drug-related affective stimuli in abstinent heroin addicts. NeuroImage. 2010;49(1):971–6.
Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99(1–3):183–92.
Yip SW, DeVito EE, Kober H, Worhunsky PD, Carroll KM, Potenza MN. Anticipatory reward processing among cocaine-dependent individuals with and without concurrent methadone-maintenance treatment: relationship to treatment response(). Drug Alcohol Depend. 2016;166:134–42.
Gradin VB, Baldacchino A, Balfour D, Matthews K, Steele JD. Abnormal brain activity during a reward and loss task in opiate-dependent patients receiving methadone maintenance therapy. Neuropsychopharmacology. 2014;39(4):885–94.
Shi Z, Wang A-L, Jagannathan K, Fairchild VP, O’Brien CP, Childress AR, et al. Effects of extended-release naltrexone on the brain response to drug-related stimuli in patients with opioid use disorder. J Psychiatry Neurosci: JPN. 2018;43(4):254–61.
Wang A-L, Lowen SB, Elman I, Shi Z, Fairchild VP, Bouril A, et al. Sustained opioid antagonism modulates striatal sensitivity to baby schema in opioid use disorder. J Subst Abus Treat. 2018;85:70–7.
Smoski MJ, Salsman N, Wang L, Smith V, Lynch TR, Dager SR, et al. Functional imaging of emotion reactivity in opiate-dependent borderline personality disorder. Personal Disord. 2011;2(3):230–41.
Schmidt A, Borgwardt S, Gerber H, Wiesbeck GA, Schmid O, Riecher-Rossler A, et al. Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress-related responses. Biol Psychiatry. 2014;76(4):289–96.
Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, et al. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438(3):322–6.
Yucel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, et al. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12(7):611 91-702.
Schmidt A, Walter M, Gerber H, Schmid O, Smieskova R, Bendfeldt K, et al. Inferior frontal cortex modulation with an acute dose of heroin during cognitive control. Neuropsychopharmacology. 2013;38(11):2231–9.
Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: lessons learned and a road ahead. NeuroImage. 2012;62(4):2281–95.
Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41.
Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11.
• Li Q, Liu J, Wang W, Wang Y, Li W, Chen J, et al. Disrupted coupling of large-scale networks is associated with relapse behaviour in heroin-dependent men. J Psychiatry Neurosci: JPN. 2018;43(1):48–57 This study investigated how resting-state functional connectivity among the salience, default mode and executive control networks correlate with heroin relapse behaviour.
Ma N, Liu Y, Li N, Wang C-X, Zhang H, Jiang X-F, et al. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49(1):738–44.
Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(Pt 7):2098–114.
Wang PW, Lin HC, Liu GC, Yang YH, Ko CH, Yen CF. Abnormal interhemispheric resting state functional connectivity of the insula in heroin users under methadone maintenance treatment. Psychiatry Res Neuroimaging. 2016;255:9–14.
Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67.
Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82.
Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15.
Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed Anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–83.
Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–74.
Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523–30.
Li Q, Li Z, Li W, Zhang Y, Wang Y, Zhu J, et al. Disrupted default mode network and basal craving in male heroin-dependent individuals: a resting-state fMRI study. J Clin Psychiatry. 2016;77(10):e1211–e7.
Li W, Li Q, Wang D, Xiao W, Liu K, Shi L, et al. Dysfunctional default mode network in methadone treated patients who have a higher heroin relapse risk. Sci Rep. 2015;5:15181.
Wang W, Wang YR, Qin W, Yuan K, Tian J, Li Q, et al. Changes in functional connectivity of ventral anterior cingulate cortex in heroin abusers. Chin Med J. 2010;123(12):1582–8.
Ma X, Qiu Y, Tian J, Wang J, Li S, Zhan W, et al. Aberrant default-mode functional and structural connectivity in heroin-dependent individuals. PLoS One. 2015;10(4):e0120861.
Yuan K, Qin W, Dong M, Liu J, Sun J, Liu P, et al. Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. Neurosci Lett. 2010;482(2):101–5.
Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, et al. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460(1):72–7.
Xie C, Shao Y, Fu L, Goveas J, Ye E, Li W, et al. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav Brain Res. 2011;216(2):639–46.
Lyoo IK, Pollack MH, Silveri MM, Ahn KH, Diaz CI, Hwang J, et al. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology. 2006;184(2):139–44.
Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, et al. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71(3):223–8.
Y-w Q, G-h J, H-h S, X-f L, J-z T, L-m L, et al. The impulsivity behavior is correlated with prefrontal cortex gray matter volume reduction in heroin-dependent individuals. Neurosci Lett. 2013;538:43–8.
Seifert CL, Magon S, Sprenger T, Lang UE, Huber CG, Denier N, et al. Reduced volume of the nucleus accumbens in heroin addiction. Eur Arch Psychiatry Clin Neurosci. 2015;265(8):637–45.
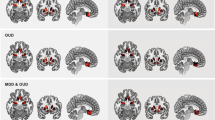
• Wollman SC, Alhassoon OM, Hall MG, Stern MJ, Connors EJ, Kimmel CL, et al. Gray matter abnormalities in opioid-dependent patients: A neuroimaging meta-analysis. The American Journal of Drug and Alcohol Abuse. 2017;43(5):505–17 This meta-analysis demonstrates that opioid-dependent individuals exhibited significantly decreased grey matter in fronto-cerebellar and fronto-insular regions compared to healthy individuals.
Wang X, Li B, Zhou X, Liao Y, Tang J, Liu T, et al. Changes in brain gray matter in abstinent heroin addicts. Drug Alcohol Depend. 2012;126(3):304–8.
Tolomeo S, Gray S, Matthews K, Steele J, Baldacchino A. Multifaceted impairments in impulsivity and brain structural abnormalities in opioid dependence and abstinence. Psychol Med. 2016;46(13):2841–53.
Qiu Y, Jiang G, Su H, Lv X, Zhang X, Tian J, et al. Progressive white matter microstructure damage in male chronic heroin dependent individuals: a DTI and TBSS study. PLoS One. 2013;8(5):e63212.
Bora E, Yücel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, et al. White matter microstructure in opiate addiction. Addict Biol. 2012;17(1):141–8.
Liu H, Li L, Hao Y, Cao D, Xu L, Rohrbaugh R, et al. Disrupted white matter integrity in heroin dependence: a controlled study utilizing diffusion tensor imaging. Am J Drug Alcohol Abuse. 2008;34(5):562–75.
Ivers JH, Fitzgerald J, Whelan C, Sweeney B, Keenan E, Fagan A, et al. Progressive white matter impairment as a predictor of outcome in a cohort of opioid-dependent patient's post-detoxification. Addict Biol. 2018;23(1):304–12.
Wollman SC, Alhassoon OM, Stern MJ, Hall MG, Rompogren J, Kimmel CL, et al. White matter abnormalities in long-term heroin users: a preliminary neuroimaging meta-analysis. Am J Drug Alcohol Abuse. 2015;41(2):133–8.
Wang Y, Li W, Li Q, Yang W, Zhu J, Wang W. White matter impairment in heroin addicts undergoing methadone maintenance treatment and prolonged abstinence: a preliminary DTI study. Neurosci Lett. 2011;494(1):49–53.
Li W, Zhu J, Li Q, Ye J, Chen J, Liu J, et al. Brain white matter integrity in heroin addicts during methadone maintenance treatment is related to relapse propensity. Brain and Behavior. 2016;6(2):e00436.
Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–8.
Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–8.
Pariyadath V, Gowin JL, Stein EA. Resting state functional connectivity analysis for addiction medicine: from individual loci to complex networks. Prog Brain Res. 2016;224:155–73.
Heilig M, Epstein DH, Nader MA, Shaham Y. Time to connect: bringing social context into addiction neuroscience. Nat Rev Neurosci. 2016;17(9):592–9.
Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185–9.
Yip SW, Scheinost D, Potenza MN, Carroll KM. Connectome-based prediction of cocaine abstinence. Am J Psychiatr. 2019. https://doi.org/10.1176/appi.ajp.2018.17101147.
Funding
This work was supported by NIDA grants T32 DA022975, K01DA039299 and R21DA045969.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Hestia Moningka, Sarah Lichenstein, and Sarah Yip declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Addictions
Rights and permissions
About this article
Cite this article
Moningka, H., Lichenstein, S. & Yip, S.W. Current Understanding of the Neurobiology of Opioid Use Disorder: an Overview. Curr Behav Neurosci Rep 6, 1–11 (2019). https://doi.org/10.1007/s40473-019-0170-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40473-019-0170-4