Abstract
Purpose of Review
Mitochondria are essential for facilitating energy homeostasis under ever-changing environmental conditions. Mitochondrial dysfunction has been suggested to either play a primary role in the development of multiple human diseases and/or significantly contribute to disease progression. Here, we review recent findings on mitochondrial dysfunction in Alzheimer’s disease (AD) and strategies for the development of mitochondria-targeted therapies.
Recent Findings
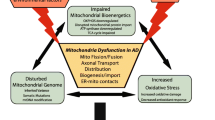
Multiple mechanisms essential for proper mitochondrial functioning including biogenesis and turnover, fission/fusion, trafficking, and bioenergetics are affected in AD. Few therapeutic strategies were developed to address these problems.
Summary
While a conclusive statement on the causative role of mitochondrial dysfunction in AD remains elusive, the available evidence suggests that improving mitochondrial function via pharmacological and/or non-pharmacological interventions could delay the onset and slow the progression of AD. Further research and outcomes of ongoing clinical trials should extend our understanding and help to validate conclusions regarding causation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17(12):865–86 Excellent review on current mitochondrial therapeutics and pathways associated with various pathologies.
Shaughnessy DT, McAllister K, Worth L, Haugen AC, Meyer JN, Domann FE, et al. Mitochondria, energetics, epigenetics, and cellular responses to stress. Environ Health Perspect. 2014;122(12):1271–8.
Quijano C, Trujillo M, Castro L, Trostchansky A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016;8:28–42.
Picard M. Mitochondrial synapses: intracellular communication and signal integration. Trends Neurosci. 2015;38(8):468–74.
Melber A, Haynes CM. UPRmt regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28(3):281–95.
Hill S, Sataranatarajan K, Remmen HV. Role of signaling molecules in mitochondrial stress response. Front Genet. 2018;9:225.
Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response. 2014;12(2):288–341.
Bárcena C, Mayoral P, Quirós PM. Mitohormesis, an antiaging paradigm. Int Rev Cell Mol Biol. 2018;340:35–77.
Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–76.
Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012;125(9):2095–104.
https://www.alz.org/alzheimers-dementia/facts-figures. This is Alzheimer's Disease Facts and Figures report published by the Alzheimer’s Association.
Talwar P, Sinha J, Grover S, Rawat C, Kushwaha S, Agarwal R, et al. Dissecting complex and multifactorial nature of Alzheimer’s disease pathogenesis: a clinical, genomic, and systems biology perspective. Mol Neurobiol. 2016;53(7):4833–64.
Deming Y, Dumitrescu L, Barnes LL, Thambisetty M, Kunkle B, Gifford KA, et al. Sex-specific genetic predictors of Alzheimer’s disease biomarkers. Acta Neuropathol. 2018;136(6):857–72
•• Swerdlow RH. Mitochondria and mitochondrial cascades in Alzheimer's disease. J Alzheimers Dis. 2018;62(3):1403–16 Current review of evidence for the mitochondrial cascade hypothesis in AD.
de la Monte SM. Type 3 diabetes is sporadic Alzheimer’s disease: mini-review. Eur Neuropsychopharmacol. 2014;24(12):1954–60.
Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008;65(9):1231–6.
Langbaum JBS, Chen K, Caselli RJ, Lee W, Reschke C, Bandy D, et al. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon 4 allele. Arch Neurol. 2010;67(4):462–8.
Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease—FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32(4):486–510.
Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–95.
Silverman DHS, Small GW, Chang CY, Lu CS, de Aburto MAK, Chen W, et al. Positron emission tomography in evaluation of dementia—regional brain metabolism and long-term outcome. JAMA. 2001;286(17):2120–7.
Iborra FJ, Kimura H, Cook PR. The functional organization of mitochondrial genomes in human cells. BMC Biol. 2004;2:9–18.
Wiesner RJ, Ruegg JC, Morano I. Counting target molecules by exponential polymerase chain-reaction - copy number of mitochondrial-DNA in rat-tissues. Biochem Biophys Res Commun. 1992;183(2):553–9.
• Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–8 Meta analysis of genetic loci associated with AD.
Swerdlow RH. Mitochondria and cell bioenergetics: increasingly recognized components and a possible etiologic cause of Alzheimer’s disease. Antioxid Redox Signal. 2012;16(12):1434–U163
Lee WT, Sun X, Tsai TS, Johnson JL, Gould JA, Garama DJ, et al. Mitochondrial DNA haplotypes induce differential patterns of DNA methylation that result in differential chromosomal gene expression patterns. Cell Death Discov. 2017;3:17062.
• Ridge PG, Kauwe JSK. Mitochondria and Alzheimer’s disease: the role of mitochondrial genetic variation. Curr Genet Med Rep. 2018;6(1):1–10 Review on mtDNA polymorphisms and haplogroups in AD.
Phillips NR, Simpkins JW, Roby RK. Mitochondrial DNA deletions in Alzheimer’s brains: a review. Alzheimer’s Dementia. 2014;10(3):393–400.
Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101(29):10726–31.
Hamblet NS, Ragland B, Ali M, Conyers B, Castora FJ. Mutations in mitochondrial-encoded cytochrome c oxidase subunits I, II, and III genes detected in Alzheimer’s disease using single-strand conformation polymorphism. Electrophoresis. 2006;27(2):398–408.
van der Walt JM, Dementieva YA, Martin ER, Scott WK, Nicodemus KK, Kroner CC, et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci Lett. 2004;365(1):28–32.
Carrieri G, Bonafe M, De Luca M, Rose G, Varcasia O, Bruni A, et al. Mitochondrial DNA haplogroups and APOE4 allele are non-independent variables in sporadic Alzheimer’s disease. Hum Genet. 2001;108(3):194–8.
Ridge PG, Koop A, Maxwell TJ, Bailey MH, Swerdlow RH, Kauwe JS, et al. Mitochondrial haplotypes associated with biomarkers for Alzheimer’s disease. PLoS One. 2013;8(9):e74158.
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. BBA Mol Basis Dis. 1802;2009:2–10.
Wang XL, Wang WZ, Li L, Perry G, Lee HG, Zhu XW. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta – Mol Basis Dis. 2014;1842(8):1240–7.
Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, et al. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol Aging. 2002;23(3):371–6.
Valla J, Berndt JD, Gonzalez-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer’s patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21(13):4923–30.
Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, et al. Brain cytochrome-oxidase in Alzheimer’s disease. J Neurochem. 1992;59(2):776–9.
Mutisya EM, Bowling AC, Beal MF. Cortical cytochrome-oxidase activity is reduced in Alzheimer’s disease. J Neurochem. 1994;63(6):2179–84.
Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21(3):455–62.
Kawamata H, Manfredi G. Proteinopathies and OXPHOS dysfunction in neurodegenerative diseases. J Cell Biol. 2017;216:3917–29.
•• Cheng Y, Bai F. The association of tau with mitochondrial dysfunction in Alzheimer’s disease. Front Neurosci. 2018;12:163 Comprehensive overview of Tau protein and its contribution to AD.
David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J Biol Chem. 2005;280(25):23802–14.
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A. 2009;106(47):20057–62.
Chowdhury SR, Djordjevic J, Albensi BC, Fernyhough P. Simultaneous evaluation of substrate-dependent oxygen consumption rates and mitochondrial membrane potential by TMRM and safranin in cortical mitochondria. Biosci Rep. 2016;36:3315.
Walls KC, Coskun P, Gallegos-Perez JL, Zadourian N, Freude K, Rasool S, et al. Swedish Alzheimer mutation induces mitochondrial dysfunction mediated by HSP60 mislocalization of amyloid precursor protein (APP) and beta-amyloid. J Biol Chem. 2012;287(36):30317–27.
Martino Adami PV, Quijano C, Magnani N, Galeano P, Evelson P, Cassina A, et al. Synaptosomal bioenergetic defects are associated with cognitive impairment in a transgenic rat model of early Alzheimer’s disease. J Cereb Blood Flow Metab. 2017;37(1):69–84.
Sonntag K-C, Ryu W-I, Amirault KM, Healy RA, Siegel AJ, McPhie DL, et al. Late-onset Alzheimer’s disease is associated with inherent changes in bioenergetics profiles. Sci Rep. 2017;7:14038.
Silva DF, Selfridge JE, Lu J, Lezi E, Roy N, Hutfles L, et al. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet. 2013;22:3931–46.
Parker WD, Filley CM, Parks JK. Cytochrome-oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40(8):1302–3.
Sims NR, Finegan JM, Blass JP. Altered glucose-metabolism in fibroblasts from patients with Alzheimer’s disease. N Engl J Med. 1985;313(10):638–9.
Perez MJ, Ponce DP, Osorio-Fuentealba C, Behrens MI, Quintanilla RA. Mitochondrial bioenergetics is altered in fibroblasts from patients with sporadic Alzheimer’s disease. Front Neurosci. 2017;11:553.
Mastroeni D, Khdour OM, Delvaux E, Nolz J, Olsen G, Berchtold N, et al. Nuclear but not mitochondrial-encoded oxidative phosphorylation genes are altered in aging, mild cognitive impairment, and Alzheimer’s disease. Alzheimer's Dementia. 2017;13:510–9.
Lunnon K, Keohane A, Pidsley R, Newhouse S, Riddoch-Contreras J, Thubron EB, et al. Mitochondrial genes are altered in blood early in Alzheimer’s disease. Neurobiol Aging. 2017;53:36–47.
Chandrasekaran K, Hatanpaa K, Rapoport SI, Brady DR. Decreased expression of nuclear and mitochondrial DNA-encoded genes of oxidative phosphorylation in association neocortex in Alzheimer disease. Mol Brain Res. 1997;44(1):99–104.
Kenney PM. Alzheimer’s disease frontal cortex mitochondria show a loss of individual respiratory proteins but preservation of respiratory complexes. In: Bennett J, editor.: Neurodegeneration Therapeutics; 2019.
Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–48.
Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1105–21.
Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128.
Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, et al. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65(5):2146–56.
Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218.
Wojsiat J, Zoltowska KM, Laskowska-Kaszub K, Wojda U. Oxidant/antioxidant imbalance in Alzheimer’s disease: therapeutic and diagnostic prospects. Oxid Med Cell Longev. 2018;2018:6435861.
Persson T, Popescu BO, Cedazo-Minguez A. Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxidative Med Cell Longev. 2014;2014:427318.
Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;71:157–76.
Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7(1):1–7.
• Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014;20(7):709–11 Review detailing mitohormesis and the involvement of ROS as a signalling molecule.
Schmeisser S, Priebe S, Groth M, Monajembashi S, Hemmerich P, Guthke R, et al. Neuronal ROS signaling rather than AMPK/sirtuin-mediated energy sensing links dietary restriction to lifespan extension. Mol Metab. 2013;2(2):92–102.
Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med. 2016;98:123–30.
Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med Cell Longev. 2016;2016:1245049.
Cagalinec M, Safiulina D, Liiv M, Liiv J, Choubey V, Wareski P, et al. Principles of the mitochondrial fusion and fission cycle in neurons. J Cell Sci. 2013;126(10):2187–97.
Cipolat S, de Brito OM, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101(45):15927–32.
Song ZY, Ghochani M, McCaffery JM, Frey TG, Chan DC. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20(15):3525–32.
Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao PZ. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011;67(1–2):103–18.
Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204(6):919–29.
Ramos ES, Larsson NG, Mourier A. Bioenergetic roles of mitochondrial fusion. Biochim Biophys Acta Bioenergetics. 2016;1857(8):1277–83.
Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH. Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta. 2010;1802(1):228–34.
• Onyango IG, Dennis J, Khan SM. Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging Dis. 2016;7:201–14 Excellent review detailing mitochondria-targeted therapeutics and mechanisms.
Yu SB, Pekkurnaz G. Mechanisms orchestrating mitochondrial dynamics for energy homeostasis. J Mol Biol. 2018;403:3922–41.
Trushina E, Nemutlu E, Zhang S, Christensen T, Camp J, Mesa J, et al. Defects in mitochondrial dynamics and metabolomic signatures of evolving energetic stress in mouse models of familial Alzheimer’s disease. PLoS One. 2012;7:e32737.
Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20(13):2495–509.
Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105(49):19318–23.
Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008;173(2):470–82.
Wang X, Su B, Lee H-G, Li X, Perry G, Smith MA, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–103.
Wang S, Song J, Tan M, Albers KM, Jia J. Mitochondrial fission proteins in peripheral blood lymphocytes are potential biomarkers for Alzheimer’s disease. Europ J Neurol. 2012;19(7):1015–22.
Bonda DJ, Wang XL, Perry G, Smith MA, Zhu XW. Mitochondrial dynamics in Alzheimer’s disease opportunities for future treatment strategies. Drugs Aging. 2010;27(3):181–92.
• Manczak M, Sesaki H, Kageyama Y, Reddy PH. Dynamin-related protein 1 heterozygote knockout mice do not have synaptic and mitochondrial deficiencies. Biochim Biophys Acta. 2012;1822(6):862–74 Studies investigating the role of mitochondria dynamic fission regulator DRP1 in promoting Aβ and Tau pathogenesis.
• Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimers disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet. 2012;21(11):2538 Studies investigating the role of mitochondria dynamic fission regulator DRP1 in promoting Aβ and Tau pathogenesis.
Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer;s disease. Hum Mol Genet. 2011;20(23):4515–29.
•• Reddy PH. Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. J Alzheimers Dis. 2014;40(2):245–56 Review describing the benefit of targeting mitochondrial dynamic regulators in AD.
• Zhang L, Trushin S, Christensen TA, Bachmeier BV, Gateno B, Schroeder A, et al. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s disease. Sci Rep. 2016;6:18725 Study describing the appearance of a new mitochondrial phenotype, MOAS or mitochondrial nanotunnels in AD.
Tyumentsev MA, Stefanova NA, Kiseleva EV, Kolosova NG. Mitochondria with morphology characteristic for Alzheimer’s disease patients are found in the brain of OXYS rats. Biochemistry-Moscow. 2018;83(9):1083–8.
Morozov YM, Datta D, Paspalas CD, Arnsten AFT. Ultrastructural evidence for impaired mitochondrial fission in the aged rhesus monkey dorsolateral prefrontal cortex. Neurobiol Aging. 2017;51:9–18.
• Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307(5713):1282–8 Impaired mitochondrial transport described as one of the key early events in AD.
Trimmer PA, Borland MK. Differentiated Alzheimer’s disease transmitochondrial cybrid cell lines exhibit reduced organelle movement. Antioxid Redox Signal. 2005;7(9–10):1101–9.
Cai Q, Tammineni P. Alterations in mitochondrial quality control in Alzheimer’s disease. Front Cell Neurosci. 2016;10:24.
• Zhang L, Trushin S, Christensen TA, Tripathi U, Hong C, Geroux RE, et al. Differential effect of amyloid beta peptides on mitochondrial axonal trafficking depends on their state of aggregation and binding to the plasma membrane. Neurobiol Dis. 2018;114:1–16 Investigating the toxicity of Aβ aggregation on mitochondrial function.
Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23(11):4499–508.
Ittner LM, Ke YD, Gotz J. Phosphorylated tau interacts with c-Jun N-terminal kinase-interacting protein 1 (JIP1) in Alzheimer disease. J Biol Chem. 2009;284(31):20909–16.
Iijima-Ando K, Sekiya M, Maruko-Otake A, Ohtake Y, Suzuki E, Lu BW, et al. Loss of axonal mitochondria promotes tau-mediated neurodegeneration and Alzheimer’s disease-related tau phosphorylation via PAR-1. Plos Genetics. 2012;8(8).
• Zhang L, Zhang S, Maezawa I, Trushin S, Minhas P, Pinto M, et al. Modulation of mitochondrial complex I activity averts cognitive decline in multiple animal models of familial Alzheimer's disease. EBioMedicine. 2015;2:294–305 Describing the use of small molecule partial inhibitors of mitochondrial complex I as a novel therapeutic strategy for AD.
Tammineni P, Jeong YY, Feng T, Aikal D, Cai Q. Impaired axonal retrograde trafficking of the retromer complex augments lysosomal deficits in Alzheimer's disease neurons. Hum Mol Genet. 2017;26(22):4352–66.
Rahman S, Archana A, Jan AT, Minakshi R. Dissecting endoplasmic reticulum unfolded protein response (UPR(ER)) in managing clandestine modus operandi of Alzheimer’s disease. Front Aging Neurosci. 2018;10:30.
•• Area-Gomez E, de Groof A, Bonilla E, Montesinos J, Tanji K, Boldogh I, et al. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018;9:335 Excellent review on the importance of mitochondrial associated membranes in AD.
Hedskog L, Pinho CM, Filadi R, Ronnback A, Hertwig L, Wiehager B, et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci U S A. 2013;110(19):7916–21.
Area-Gomez E, Castillo MDL, Tambini MD, Guardia-Laguarta C, de Groof AJC, Madra M, et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31(21):4106–23.
Pera M, Larrea D, Guardia-Laguarta C, Montesinos J, Velasco KR, Agrawal RR, et al. Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease. EMBO J. 2017;36(22):3356–71.
Yu J, Novgorodov SA, Chudakova D, Zhu H, Bielawska A, Bielawski J, et al. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J Biol Chem. 2007;282(35):25940–9.
Monette JS, Gomez LA, Moreau RF, Dunn KC, Butler JA, Finlay LA, et al. (R)-alpha-lipoic acid treatment restores ceramide balance in aging rat cardiac mitochondria. Pharmacol Res. 2011;63(1):23–9.
Trounce IA, Crouch PJ, Carey KT, McKenzie M. Modulation of ceramide-induced cell death and superoxide production by mitochondrial DNA-encoded respiratory chain defects in Rattus xenocybrid mouse cells. BBA-Bioenergetics. 2013;1827(7):817–25.
Zigdon H, Kogot-Levin A, Park JW, Goldschmidt R, Kelly S, Merrill AH Jr, et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J Biol Chem. 2013;288:4947–56.
Kogot-Levin A, Saada A. Ceramide and the mitochondrial respiratory chain. Biochimie. 2014;100:88–94.
Ji WK, Chakrabarti R, Fan XT, Schoenfeld L, Strack S, Higgs HN. Receptor-mediated Drp1 oligomerization on endoplasmic reticulum. J Cell Biol. 2017;216(12):4123–39.
Sorrentino V, Omani MR, Ouchiroud LM, Beck JS, Zhang HB, D'Amico D, et al. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature. 2017;552(7684):187–93.
Beck JS, Mufson EJ, Counts SE. Evidence for mitochondrial UPR gene activation in familial and sporadic Alzheimer’s disease. Curr Alzheimer Res. 2016;13(6):610–4.
Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, et al. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome, PreP. J Biol Chem. 2006;281(39):29096–104.
Alikhani N, Guo L, Yan SQ, Du H, Pinho CM, Chen JX, et al. Decreased proteolytic activity of the mitochondrial amyloid-beta degrading enzyme, PreP peptidasome, in Alzheimer’s disease brain mitochondria. J Alzheimers Dis. 2011;27(1):75–87.
Gregori L, Fuchs C, Figueiredopereira ME, Vannostrand WE, Goldgaber D. Amyloid beta-protein inhibits ubiquitin-dependent protein-degradation in-vitro. J Biol Chem. 1995;270(34):19702–8.
Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. J Neurochem. 2000;75(1):436–9.
Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM. A beta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol Aging. 2008;29(11):1607–18.
• Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20(9):1013–22 Excellent review on cellular quality control mechanisms.
Martin-Maestro P, Gargini R, Perry G, Avila J, Garcia-Escudero V. PARK2 enhancement is able to compensate mitophagy alterations found in sporadic Alzheimer's disease. Hum Mol Genet. 2016;25(4):792–806.
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64(2):113–22.
Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(23):4081–91.
Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, et al. Macroautophagy—a novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171(1):87–98.
Bordi M, Berg MJ, Mohan PS, Peterhoff CM, Alldred MJ, Che SL, et al. Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy. 2016;12(12):2467–83.
Ye X, Sun X, Starovoytov V, Cai Q. Parkin-mediated mitophagy in mutant hAPP neurons and Alzheimer’s disease patient brains. Hum Mol Genet. 2015.
Reddy AP, Reddy PH. Mitochondria-targeted molecules as potential drugs to treat patients with Alzheimer’s disease. Prog Mol Biol Transl Sci. 2017;146:173–201.
Hara Y, McKeehan N, Fillit HM. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology. 2019;92(2):84–93.
Rotermund C, Machetanz G, Fitzgerald JC. The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol (Lausanne). 2018;9:400.
Ribarič S. The rationale for insulin therapy in Alzheimer’s disease. Molecules. 2016;21(6).
Jardim FR, de Rossi FT, Nascimento MX, da Silva Barros RG, Borges PA, Prescilio IC, et al. Resveratrol and brain mitochondria: a review. Mol Neurobiol. 2018;55(3):2085–101.
Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, et al. The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell. 2018;17(2).
Komaki H, Faraji N, Komaki A, Shahidi S, Etaee F, Raoufi S, Mirzaei F. Investigation of protective effects of coenzyme Q10 on impaired synaptic plasticity in a male rat model of Alzheimer's disease. Brain Res Bull. 2019;9:(147):14–21.
Kumar A, Singh A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front Pharmacol. 2015;6:206.
Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14(2):193–204.
Christ MG, Huesmann H, Nagel H, Kern A, Behl C. Sigma-1 receptor activation induces autophagy and increases proteostasis capacity in vitro and in vivo. Cells. 2019;8(3).
Hebron ML, Lonskaya I, Moussa CE. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson’s disease models. Hum Mol Genet. 2013;22(16):3315–28.
Li W, Huang E. An update on type 2 diabetes mellitus as a risk factor for dementia. J Alzheimers Dis. 2016;53(2):393–402.
Searcy JL, Phelps JT, Pancani T, Kadish I, Popovic J, Anderson KL, et al. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2012;30(4):943–61.
Seok H, Lee M, Shin E, Yun MR, Lee YH, Moon JH, et al. Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci Rep. 2019;9(1):4414.
Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199(2):265–73.
Escribano L, Simón AM, Pérez-Mediavilla A, Salazar-Colocho P, Del Río J, Frechilla D. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer’s disease mouse model. Biochem Biophys Res Commun. 2009;379(2):406–10.
Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol Psychiatry. 2010;15(3):272–85 28.
O’Reilly JA, Lynch M. Rosiglitazone improves spatial memory and decreases insoluble Aβ(1-42) in APP/PS1 mice. J Neuroimmune Pharmacol. 2012;7(1):140–4.
Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis. 2007;11(1):45–51.
Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54(5):1392–9.
Ghosh S, Patel N, Rahn D, McAllister J, Sadeghi S, Horwitz G, et al. The thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Mol Pharmacol. 2007;71(6):1695–702.
Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30(2):131–46.
Koenig AM, Mechanic-Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L, et al. Effects of the insulin sensitizer metformin in Alzheimer disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord. 2017;31(2):107–13.
Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, et al. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: a pilot clinical trial. J Alzheimers Dis. 2017;57(4):1325–34.
Zhong KL, Chen F, Hong H, Ke X, Lv YG, Tang SS, et al. New views and possibilities of antidiabetic drugs in treating and/or preventing mild cognitive impairment and Alzheimer’s disease. Metab Brain Dis. 2018;33(4):1009–18.
Gomes BAQ, Silva JPB, Romeiro CFR, Dos Santos SM, Rodrigues CA, Gonçalves PR, et al. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: role of SIRT1. Oxidative Med Cell Longev. 2018;2018:8152373.
Madrigal-Perez LA, Ramos-Gomez M. Resveratrol inhibition of cellular respiration: new paradigm for an old mechanism. Int J Mol Sci. 2016;17(3):368.
Sawda C, Moussa C, Turner RS. Resveratrol for Alzheimer’s disease. Ann N Y Acad Sci. 2017;1403(1):142–9.
Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822(5):639–49.
Feniouk BA, Skulachev VP. Cellular and molecular mechanisms of action of mitochondria-targeted antioxidants. Curr Aging Sci. 2017;10:41–8.
Reddy PH, Manczak M, Yin X. Mitochondria-division inhibitor 1 protects against amyloid-β induced mitochondrial fragmentation and synaptic damage in Alzheimer’s disease. J Alzheimers Dis. 2017;58(1):147–62.
Reddy PH, Manczak M, Yin X, Reddy AP. Synergistic protective effects of mitochondrial division inhibitor 1 and mitochondria-targeted Small peptide SS31 in Alzheimer's disease. J Alzheimers Dis. 2018;62(4):1549–65.
Wang W, Yin J, Ma X, Zhao F, Siedlak SL, Wang Z, et al. Inhibition of mitochondrial fragmentation protects against Alzheimer’s disease in rodent model. Hum Mol Genet. 2017;26:4118–31.
Baek SH, Park SJ, Jeong JI, Kim SH, Han J, Kyung JW, et al. Inhibition of Drp1 ameliorates synaptic depression, Aβ deposition, and cognitive impairment in an Alzheimer’s disease model. J Neurosci. 2017;37(20):5099–110.
Smith G, Gallo G. To mdivi-1 or not to mdivi-1: is that the question? Dev Neurobiol. 2017;77(11):1260–8.
Lonskaya I, Hebron ML, Desforges NM, Franjie A, Moussa CE. Tyrosine kinase inhibition increases functional Parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol Med. 2013;5(8):1247–62.
Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–26.
Bertram S, Brixius K, Brinkmann C. Exercise for the diabetic brain: how physical training may help prevent dementia and Alzheimer’s disease in T2DM patients. Endocrine. 2016;53(2):350–63.
Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22(2):569–79.
Mendiola-Precoma J, Berumen LC, Padilla K, Garcia-Alcocer G. Therapies for prevention and treatment of Alzheimer’s disease. Biomed Res Int. 2016;2016:2589276.
Ntsapi C, Loos B. Caloric restriction and the precision-control of autophagy: a strategy for delaying neurodegenerative disease progression. Exp Gerontol. 2016;83:97–111.
Van Cauwenberghe C, Vandendriessche C, Libert C, Vandenbroucke RE. Caloric restriction: beneficial effects on brain aging and Alzheimer’s disease. Mamm Genome. 2016;27(7–8):300–19.
•• Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–63 Current investigative trials on modifiable risk factors for AD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- UPRmt
-
mitochondrial unfolded protein response
- 3D EM
-
three-dimensional electron microscopy;
- 3-NT
-
3-nitrotyrosine, a product of nitrosative stress in cells;
- AD
-
Alzheimer’s disease;
- AMPK
-
Adenosine Monophosphate (AMP)-activated protein kinase;
- Anavex 2-73
-
tetrahydro-N,N-dimethyl-2,2-diphenyl-3-furanmethanamine;
- APP
-
amyloid precursor protein;
- ARE
-
antioxidant response element, which plays a critical role in the protection against oxidative stress and maintenance of redox homeostasis;
- ATP
-
adenosine triphosphate;
- Aβ
-
amyloid-β peptide;
- Bioenergetics
-
a biochemical process of cellular energy production, storage and utilization;
- CT1812
-
sigma-2 receptor complex allosteric antagonist;
- Cybrids
-
cell lines that maintain the same nuclear DNA but differ in mitochondrial DNA;
- DRP1
-
Dynamin-related protein 1;
- ER
-
endoplasmic reticulum;
- ETC
-
electron transport chain consisting of mitochondrial complexes I–IV that maintain electron flow and proton gradient essential for the ATP production by mitochondrial complex V;
- FDA
-
the US Food and Drug Administration;
- FDG-PET
-
fluorodeoxyglucose-positron emission tomography;
- GPX
-
glutathione peroxidase;
- GSK-3β
-
Glycogen synthase kinase 3 beta;
- GTPases
-
a large family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP);
- HNE
-
4-hydroxyl-2-trans-nonenal, a product of oxidative stress in cells;
- IMM
-
inner mitochondrial membranes;
- JIP1
-
c-Jun N-terminal kinase-interacting protein 1;
- MAM
-
mitochondria-associated membranes;
- MCI
-
mild cognitive impairment, a prodromal stage of AD;
- Mdivi-1
-
Mitochondrial division inhibitor 1;
- MFN1
-
Mitofusin 1 protein;
- MFN2
-
Mitofusin 2 protein;
- Mitochondrial biogenesis
-
the regulated process that increases mitochondrial mass;
- Mitochondrial fission
-
the ability of organelles to divide in response to environmental changes;
- Mitochondrial fusion
-
the ability of organelles to fuse together in response to environmental changes;
- Mitochondrial haplotypes
-
specific regions of mtDNA that cluster with other mitochondrial sequences to show the phylogenetic origins of maternal lineages;
- Mitohormesis
-
the mitochondrial signaling via ROS molecules that promotes health by preventing or delaying a number of chronic diseases;
- Mitophagy
-
the process of removal of damages organelles by lysosomal degradation;
- MitoQ
-
mitoquinone mesylate;
- MitoTEMPOL
-
mitochondria-targeting superoxide dismutase mimetic;
- MitoVitE
-
mitotocopherol;
- MOAS
-
mitochondria-on-a-string;
- mtDNA
-
mitochondrial DNA;
- NADH
-
nicotinamide adenine dinucleotide;
- Nrf-1and Nrf-2
-
nuclear factor erythroid-derived 2-related factor 1 and 2;
- OMM
-
outer mitochondrial membranes;
- OPA1
-
Optic atrophy 1 protein;
- OXPHOS complexes
-
complex I (nicotinamide adenine dinucleotide [NADH] dehydrogenase), complex II (succinate dehydrogenase), complex III (cytochrome C reductase), complex IV (cytochrome C oxidase [COX]), and complex V (ATP synthase);
- OXPHOS
-
oxidative phosphorylation;
- Parkin
-
an E3 ubiquitin ligase that promotes the covalent ubiquitination of molecules tagging them for degradation in proteasomes or lysosomes;
- Pink1
-
PTEN-induced kinase 1, a mitochondrial serine/threonine-protein kinase encoded by the PINK1 gene;
- PreP
-
pre-sequence protease;
- PS1
-
presenilin 1;
- PS2
-
presenilin 2, mutations in APP, PS1 and PS2 proteins, all of which are involved in processing of amyloid beta peptides, are linked to familial AD;
- pTau
-
phospho Tau protein;
- Redox state
-
the ratio of the interconvertible oxidized and reduced form of a specific redox couple;
- RNS
-
reactive nitrogen species;
- ROS
-
reactive oxygen species;
- SNPs
-
single nucleotide polymorphisms are variations in a single base pair in a DNA sequence;
- SOD
-
superoxide dismutase;
- SS peptides
-
Szeto-Schiller peptide antioxidants;
- Synaptosomes
-
an enriched fraction of synaptic proteins and synaptic mitochondria isolated from neurons or brain tissue;
- TCA
-
tricarboxylic acid cycle
Rights and permissions
About this article
Cite this article
Flannery, P.J., Trushina, E. Mitochondrial Dysfunction in Alzheimer’s Disease and Progress in Mitochondria-Targeted Therapeutics. Curr Behav Neurosci Rep 6, 88–102 (2019). https://doi.org/10.1007/s40473-019-00179-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40473-019-00179-0