Abstract
Purpose of Review
Highly palatable foods (HPF) have rewarding effects, and their consumption induces gut dysbiosis. Because intestinal microbes communicate bidirectionally with the brain, we reviewed the literature in order to link the effects of HPF on the brain reward system and on gut microbiota. Additionally, we propose these alterations contribute to the pathophysiology of obesity.
Recent Findings
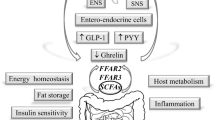
Non-homeostatic consumption of HPF programs the brain to seek these foods from early-life. Fatty food induces gut dysbiosis, which might alter communications to the brain. Additionally, prebiotic fibre and short-chain fatty acids affect the neurochemistry of the rodent mesocorticolimbic circuit.
Summary
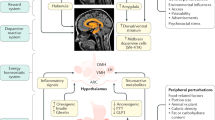
Consumption of HPF might start a vicious cycle by (1) activating the mesocorticolimbic circuit, leading to (2) non-homeostatic feeding, affecting the host’s metabolism and (3) altering gut microbes. The latter might impact the brain’s reward system, which becomes reinforced by signals from gut symbionts, thus contributing to the pathophysiology of obesity.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Adlerberth I, Carlsson B, de Man P, Jalil F, Khan SR, Larsson P, et al. Intestinal colonization with Enterobacteriaceae in Pakistani and Swedish hospital-delivered infants. Acta Paediatr Scand. 1991;80(6–7):602–10.
Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. https://doi.org/10.3389/fphys.2011.00094.
Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9(10):565–76. https://doi.org/10.1038/nrgastro.2012.144.
Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–79. https://doi.org/10.1056/NEJMra1600266.
Ge X, Zhao W, Ding C, Tian H, Xu L, Wang H, et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci Rep. 2017;7(1):441. https://doi.org/10.1038/s41598-017-00612-y.
Luczynski P, Tramullas M, Viola M, Shanahan F, Clarke G, O'Mahony S, et al. Microbiota regulates visceral pain in the mouse. elife. 2017;6. https://doi.org/10.7554/eLife.25887.
Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–52. https://doi.org/10.1073/pnas.1010529108.
Wang ZW, Wang P, Lin FH, Li XL, Li XF, O'Byrne KT, et al. Early-life exposure to lipopolysaccharide reduces the severity of experimental autoimmune encephalomyelitis in adulthood and correlated with increased urine corticosterone and apoptotic CD4+ T cells. Neuroscience. 2011;193:283–90. https://doi.org/10.1016/j.neuroscience.2011.07.047.
Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509–18. https://doi.org/10.1016/j.molmed.2014.05.002.
Clarke G, O'Mahony SM, Dinan TG, Cryan JF. Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr. 2014;103(8):812–9. https://doi.org/10.1111/apa.12674.
Ogbonnaya ES, Clarke G, Shanahan F, Dinan TG, Cryan JF, O'Leary OF. Adult hippocampal neurogenesis is regulated by the microbiome. Biol Psychiatry. 2015;78(4):e7–9. https://doi.org/10.1016/j.biopsych.2014.12.023.
Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19(2):146–8. https://doi.org/10.1038/mp.2013.65.
Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–17. https://doi.org/10.1136/gut.2009.202515.
Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18. https://doi.org/10.1016/j.jpsychires.2016.07.019.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Mol Psychiatry. 2016;21(6):786–96. https://doi.org/10.1038/mp.2016.44.
Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–73. https://doi.org/10.1038/mp.2012.77.
De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017;9(379) https://doi.org/10.1126/scitranslmed.aaf6397.
Bravo JA, Julio-Pieper M, Forsythe P, Kunze W, Dinan TG, Bienenstock J, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12(6):667–72. https://doi.org/10.1016/j.coph.2012.09.010.
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167(6):1469–80 e12. https://doi.org/10.1016/j.cell.2016.11.018.
Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep. 2016;6:30028. https://doi.org/10.1038/srep30028.
Fallon J. Could one of the most widely prescribed antibiotics amoxicillin/clavulanate "augmentin" be a risk factor for autism? Med Hypotheses. 2005;64(2):312–5. https://doi.org/10.1016/j.mehy.2004.06.023.
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–63. https://doi.org/10.1016/j.cell.2013.11.024.
Slykerman RF, Thompson J, Waldie KE, Murphy R, Wall C, Mitchell EA. Antibiotics in the first year of life and subsequent neurocognitive outcomes. Acta Paediatr. 2017;106(1):87–94. https://doi.org/10.1111/apa.13613.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. https://doi.org/10.1038/npp.2009.110.
Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–25.
Frohmader KS, Pitchers KK, Balfour ME, Coolen LM. Mixing pleasures: review of the effects of drugs on sex behavior in humans and animal models. Horm Behav. 2010;58(1):149–62. https://doi.org/10.1016/j.yhbeh.2009.11.009.
Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30(8):375–81. https://doi.org/10.1016/j.tins.2007.06.004.
Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17(2):851–61.
Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. https://doi.org/10.1146/annurev.neuro.30.051606.094341.
Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–8.
Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–13. https://doi.org/10.1176/appi.ajp.162.8.1403.
Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87(17):6912–6.
Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2(2):119–28. https://doi.org/10.1038/35053570.
Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98(20):11042–6. https://doi.org/10.1073/pnas.191352698.
Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. https://doi.org/10.1016/0149-7634(95)00033-B.
Gibbons C, Blundell J. Appetite regulation and physical activity - an energy balance perspective. Hamdan Med J. 2015;8(1):33–52. https://doi.org/10.7707/hmj.431.
Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD. Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology. 2008;197(1):83–94. https://doi.org/10.1007/s00213-007-1008-4.
Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151(10):4756–64. https://doi.org/10.1210/en.2010-0505.
Esteve E, Ricart W, Fernandez-Real JM. Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: did gut microbiote co-evolve with insulin resistance? Curr Opin Clin Nutr Metab Care. 2011;14(5):483–90. https://doi.org/10.1097/MCO.0b013e328348c06d.
Valsecchi C, Carlotta Tagliacarne S, Castellazzi A. Gut Microbiota and Obesity. J Clin Gastroenterol. 2016;50 Suppl 2, Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13–15, 2015:S157-S8. https://doi.org/10.1097/MCG.0000000000000715
Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. 2016;2016:7353642–27. https://doi.org/10.1155/2016/7353642.
Duranti S, Ferrario C, van Sinderen D, Ventura M, Turroni F. Obesity and microbiota: an example of an intricate relationship. Genes Nutr. 2017;12:18. https://doi.org/10.1186/s12263-017-0566-2.
Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9(10):577–89. https://doi.org/10.1038/nrgastro.2012.156.
Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36. https://doi.org/10.1042/BCJ20160510.
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar RD. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–803. https://doi.org/10.3748/wjg.v21.i29.8787.
• Brahe LK, Astrup A, Larsen LH. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv Nutr. 2016;7(1):90–101. https://doi.org/10.3945/an.115.010587 This review provides a nice summary of evidence from studies associating gut microbiota and obesity or type 2 diabetes, as well as reports on the effects of dietary intervention aimed to alter the gut microbiota in metabolic disease prevention and treatment.
Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2011;22(8):712–22. https://doi.org/10.1016/j.jnutbio.2010.05.009.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. https://doi.org/10.1038/nature12506.
Zhang P, Meng X, Li D, Calderone R, Mao D, Sui B. Commensal homeostasis of gut microbiota-host for the impact of obesity. Front Physiol. 2017;8:1122. https://doi.org/10.3389/fphys.2017.01122.
• Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, et al. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci Rep. 2016;6:35455. https://doi.org/10.1038/srep35455 The change in sensitivity towards cocaine after 2 weeks of treatment with an oral cocktail of wide spectrum antibiotics, an effect that is reverted by short-chain fatty acids, is a major finding linking the effects of intestinal dysbiosis with changes on the mesocorticolimbic circuit in mice. This evidence highlight that changes in gut microbes affect certain types of behaviours. This work proposes novel targets in the field of addiction.
Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. https://doi.org/10.1053/j.gastro.2011.04.052.
van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O'Sullivan O, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018. https://doi.org/10.1113/JP276431.
•• Byrne CS, Chambers ES, Alhabeeb H, Chhina N, Morrison DJ, Preston T, et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr. 2016;104(1):5–14. https://doi.org/10.3945/ajcn.115.126706 This work links the effects of bacterial metabolites on the mesocorticolimbic system in healthy volunteers. Furthermore, it suggests that the mesocorticolimbic system is modifiable through changes in gut microbes in non-obese, healthy volunteers, through dietary supplements.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491. https://doi.org/10.1038/nrgastro.2017.75.
Hadri Z, Chaumontet C, Fromentin G, Even PC, Darcel N, Bouras AD, et al. Long term ingestion of a preload containing fructo-oligosaccharide or guar gum decreases fat mass but not food intake in mice. Physiol Behav. 2015;147:198–204. https://doi.org/10.1016/j.physbeh.2015.04.039.
Bindels LB, Neyrinck AM, Salazar N, Taminiau B, Druart C, Muccioli GG, et al. Non digestible oligosaccharides modulate the gut microbiota to control the development of leukemia and associated cachexia in mice. PLoS One. 2015;10(6):e0131009. https://doi.org/10.1371/journal.pone.0131009.
Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res. 2005;13(6):1000–7. https://doi.org/10.1038/oby.2005.117.
Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr. 2005;60:567–72. https://doi.org/10.1038/sj.ejcn.1602350.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci. 2013;110(22):9066–71. https://doi.org/10.1073/pnas.1219451110.
McLean PG, Bergonzelli GE, Collins SM, Bercik P. Targeting the microbiota-gut-brain axis to modulate behavior: which bacterial strain will translate best to humans? Proc Natl Acad Sci U S A. 2012;109(4):E174; author reply E6. https://doi.org/10.1073/pnas.1118626109.
Burokas A, Moloney RD, Dinan TG, Cryan JF. Microbiota regulation of the mammalian gut-brain axis. Adv Appl Microbiol. 2015;91:1–62. https://doi.org/10.1016/bs.aambs.2015.02.001.
Messaoudi M, Rozan P, Nejdi A, Hidalgo S, Desor D. Behavioural and cognitive effects of oligofructose-enriched inulin in rats. Br J Nutr. 2005;93(S1):S27–30. https://doi.org/10.1079/BJN20041348.
•• Delbes AS, Castel J, Denis RGP, Morel C, Quinones M, Everard A, et al. Prebiotics supplementation impact on the reinforcing and motivational aspect of feeding. Front Endocrinol (Lausanne). 2018;9:273. https://doi.org/10.3389/fendo.2018.00273 This work strongly suggest that a high-sugar high-fat diet induces gut dysbiosis, and moreover, addition of the prebiotic FOS induces different effects depending if FOS is given while the animals are consuming the high-energy diet, or after the period of high-energy diet consumption. The importance here is that the effects on the brain are specific, and that these effects depend on the type of bacteria inhabiting the intestinal milieu.
Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M. The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis. 2016;15:108. https://doi.org/10.1186/s12944-016-0278-4.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. https://doi.org/10.1038/4441022a.
Okeke F, Roland BC, Mullin GE. The role of the gut microbiome in the pathogenesis and treatment of obesity. Glob Adv Health Med. 2014;3(3):44–57. https://doi.org/10.7453/gahmj.2014.018.
Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. https://doi.org/10.1186/s12876-015-0330-2.
• Haro C, Montes-Borrego M, Rangel-Zuniga OA, Alcala-Diaz JF, Gomez-Delgado F, Perez-Martinez P, et al. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J Clin Endocrinol Metab. 2016;101(1):233–42. https://doi.org/10.1210/jc.2015-3351 This investigation on long-term consumption of the Mediterranean diet and low-fat, high-complex carbohydrate (LFHCC) diet very neatly shows their protective effect on the development of type 2 diabetes by exerting specific changes in the gut microbiota. It highlights the role of gut microbes in mediating the therapeutic effects of diet in human health.
Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 2011;19(7):349–59. https://doi.org/10.1016/j.tim.2011.05.006.
Sanmiguel C, Gupta A, Mayer EA. Gut microbiome and obesity: a plausible explanation for obesity. Curr Obes Rep. 2015;4(2):250–61. https://doi.org/10.1007/s13679-015-0152-0.
Conterno L, Fava F, Viola R, Tuohy KM. Obesity and the gut microbiota: does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011;6(3):241–60. https://doi.org/10.1007/s12263-011-0230-1.
Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda). 2016;31(4):283–93. https://doi.org/10.1152/physiol.00041.2015.
Funding
This work was supported by FONDECYT no. 1140776 and no. 1181019, IDRC.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Brain and Microbiome
Rights and permissions
About this article
Cite this article
Urrutia-Piñones, J., Illanes-González, J., López-Aguilera, A. et al. Do Obese Bacteria Make us “Want them”? Intestinal Microbiota, Mesocorticolimbic Circuit and Non-Homeostatic Feeding. Curr Behav Neurosci Rep 5, 211–217 (2018). https://doi.org/10.1007/s40473-018-0161-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40473-018-0161-x