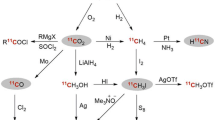
Abstract
Purpose
Copper-mediated radiofluorination (CMRF) is emerging as the method of choice for the formation of aromatic C–18F bonds. This minireview examines proof-of-concept, preclinical, and in-human imaging studies of new and established imaging agents containing aromatic C–18F bonds synthesized with CMRF. An exhaustive discussion of CMRF methods is not provided, although key developments that have enabled or improved upon the syntheses of fluorine-18 imaging agents are discussed.
Methods
A comprehensive literature search from April 2014 onwards of the Web of Science and PubMed library databases was performed to find reports that utilize CMRF for the synthesis of fluorine-18 radiopharmaceuticals, and these represent the primary body of research discussed in this minireview. Select conference proceedings, previous reports describing alternative methods for the synthesis of imaging agents, and preceding fluorine-19 methodologies have also been included for discussion.
Conclusions
CMRF has significantly expanded the chemical space that is accessible to fluorine-18 radiolabeling with production methods that can meet the regulatory requirements for use in Nuclear Medicine. Furthermore, it has enabled novel and improved syntheses of radiopharmaceuticals and facilitated subsequent PET imaging studies. The rapid adoption of CMRF will undoubtedly continue to simplify the production of imaging agents and inspire the development of new radiofluorination methodologies.




































Similar content being viewed by others
References
Ametamey SM, Honer M, Schubiger PA (2008) Molecular Imaging with PET. Chem Rev 108:1501–1516. https://doi.org/10.1021/cr0782426
Pither R (2003) PET and the role of in vivo molecular imaging in personalized medicine. Expert Rev Mol Diagn 3:703–713. https://doi.org/10.1586/14737159.3.6.703
Van Der Veldt AAM, Lubberink M, Greuter HN, Comans EFI, Herder GJM, Yaqub M, Schuit RC, Van Lingen A, Rizvi SN, Mooijer MPJ, Rijnders AY, Windhorst AD, Smit EF, Hendrikse NH, Lammertsma AA (2011) Absolute quantification of [11C]docetaxel kinetics in lung cancer patients using positron emission tomography. Clin Cancer Res 17:4814–4824. https://doi.org/10.1158/1078-0432.CCR-10-2933
Avril NE, Weber WA (2005) Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am 43:189–204. https://doi.org/10.1016/j.rcl.2004.09.006
Sevigny J, Suhy J, Chiao P, Chen T, Klein G, Purcell D, Oh J, Verma A, Sampat M, Barakos J (2016) Amyloid PET screening for enrichment of early-stage alzheimer disease clinical trials: experience in a phase 1b clinical trial. Alzheimer Dis Assoc Disord 30:1–7. https://doi.org/10.1097/WAD.0000000000000144
Matthews PM, Rabiner EA, Passchier J, Gunn RN (2012) Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol 73:175–186. https://doi.org/10.1111/j.1365-2125.2011.04085.x
Elsinga P, van Waarde A, Paans A, Dierckx R (2012) Trends on the Role of PET in Drug Development. Worldwide Scientific, Singapore
Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA (2015) Applications of fluorine in medicinal chemistry. J Med Chem 58:8315–8359. https://doi.org/10.1021/acs.jmedchem.5b00258
Ducharme J, Goertzen AL, Patterson J, Demeter S (2009) Practical aspects of 18F-FDG PET when receiving 18F-FDG from a distant supplier. J Nucl Med Technol 37:164–169. https://doi.org/10.2967/jnmt.109.062950
Deng X, Rong J, Wang L, Vasdev N, Zhang L, Josephson L, Liang SH (2019) Chemistry for positron emission tomography: recent advances in 11C-, 18F-, 13 N-, and 15O-labeling reactions. Angew Chemie Int Ed 58:2580–2605. https://doi.org/10.1002/anie.201805501
Brooks AF, Drake LR, Stewart MN, Cary BP, Jackson IM, Mallette D, Mossine AV, Scott PJH (2016) Fluorine-18 patents (2009–2015). part 1: novel radiotracers. Pharm Pat Anal 5:17–47. https://doi.org/10.4155/ppa.15.36
Mossine AV, Thompson S, Brooks AF, Sowa AR, Miller JM, Scott PJH (2016) Fluorine-18 patents (2009–2015). Part 2: new radiochemistry. Pharm Pat Anal 5:319–349. https://doi.org/10.4155/ppa-2016-0028
Brooks AF, Topczewski JJ, Ichiishi N, Sanford MS, Scott PJH (2014) Late-stage [18F]fluorination: new solutions to old problems. Chem Sci 5:4545–4553. https://doi.org/10.1039/C4SC02099E
Tredwell M, Gouverneur V (2012) 18F Labeling of arenes. Angew Chemie Int Ed 51:11426–11437. https://doi.org/10.1002/anie.201204687
Van Der Born D, Pees A, Poot AJ, Orru RVA, Windhorst AD, Vugts DJ (2017) Fluorine-18 labelled building blocks for PET tracer synthesis. Chem Soc Rev 46:4709–4773. https://doi.org/10.1039/c6cs00492j
Sanford MS, Scott PJH (2016) Moving metal-mediated 18F-fluorination from concept to clinic. ACS Cent Sci 2:128–130. https://doi.org/10.1021/acscentsci.6b00061
Preshlock S, Tredwell M, Gouverneur V (2016) 18F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem Rev 116:719–766. https://doi.org/10.1021/acs.chemrev.5b00493
Thompson S, Lee SJ, Jackson IM, Ichiishi N, Brooks AF, Sanford MS, Scott PJH (2019) Synthesis of [18F]-γ-fluoro-α, β-unsaturated esters and ketones via vinylogous 18F-fluorination of α-diazoacetates with [18 F]AgF. Synth 51:4401–4407. https://doi.org/10.1055/s-0039-1690012
Lee SJ, Brooks AF, Ichiishi N, Makaravage KJ, Mossine AV, Sanford MS, Scott PJH (2019) C–H 18F-fluorination of 8-methylquinolines with Ag[18F]F. Chem Commun 55:2976–2979. https://doi.org/10.1039/C9CC00641A
Gray EE, Nielsen MK, Choquette KA, Kalow JA, Graham TJA, Doyle AG (2016) Nucleophilic (Radio)fluorination of α-diazocarbonyl compounds enabled by copper-catalyzed H-F insertion. J Am Chem Soc 138:10802–10805. https://doi.org/10.1021/jacs.6b06770
Beyzavi MH, Mandal D, Strebl MG, Neumann CN, D’Amato EM, Chen J, Hooker JM, Ritter T, D’Amato EM, Chen J, Hooker JM, Ritter T (2017) 18F-Deoxyfluorination of phenols via Ru π-complexes. ACS Cent Sci 3:944–948. https://doi.org/10.1021/acscentsci.7b00195
Hoover AJ, Lazari M, Ren H, Narayanam MK, Murphy JM, Van Dam RM, Hooker JM, Ritter T (2016) A transmetalation reaction enables the synthesis of [18F]5-fluorouracil from [18F]fluoride for human PET imaging. Organometallics 35:1008–1014. https://doi.org/10.1021/acs.organomet.6b00059
Brandt JR, Lee E, Boursalian GB, Ritter T (2014) Mechanism of electrophilic fluorination with Pd(IV): fluoride capture and subsequent oxidative fluoride transfer. Chem Sci 5:169–179. https://doi.org/10.1039/c3sc52367e
Verhoog S, Brooks AF, Winton WP, Viglianti BL, Sanford MS, Scott PJH (2019) Ring opening of epoxides with [18F]FeF species to produce [18F]fluorohydrin PET imaging agents. Chem Commun. https://doi.org/10.1039/C9CC02779C
Ichiishi N, Canty AJ, Yates BF, Sanford MS (2013) Cu-catalyzed fluorination of diaryliodonium salts with KF. Org Lett 15:5134–5137. https://doi.org/10.1021/ol4025716
Ye Y, Schimler SD, Hanley PS, Sanford MS (2013) Cu(OTf)2-mediated fluorination of aryltrifluoroborates with potassium fluoride. J Am Chem Soc 135:16292–16295. https://doi.org/10.1021/ja408607r
Gamache RF, Waldmann C, Murphy JM (2016) Copper-mediated oxidative fluorination of aryl stannanes with fluoride. Org Lett 18:4522–4525. https://doi.org/10.1021/acs.orglett.6b02125
Fier PS, Luo J, Hartwig JF (2013) Copper-mediated fluorination of arylboronate esters. identification of a copper(III) fluoride complex. J Am Chem Soc 135:2552–2559. https://doi.org/10.1021/ja310909q
Fier PS, Hartwig JF (2012) Copper-mediated fluorination of aryl iodides. J Am Chem Soc 134:10795–10798. https://doi.org/10.1021/ja304410x
Mu X, Zhang H, Chen P, Liu G (2014) Copper-catalyzed fluorination of 2-pyridyl aryl bromides. Chem Sci 5:275–280. https://doi.org/10.1039/c3sc51876k
Tredwell M, Preshlock SM, Taylor NJ, Gruber S, Huiban M, Passchier J, Mercier J, Génicot C, Gouverneur V (2014) A general copper-mediated nucleophilic 18F fluorination of arenes. Angew Chem Int Ed 53:7751–7755. https://doi.org/10.1002/anie.201404436
Ichiishi N, Brooks AF, Topczewski JJ, Rodnick ME, Sanford MS, Scott PJH (2014) Copper-catalyzed [18F]fluorination of (Mesityl)(aryl)iodonium salts. Org Lett 16:3224–3227. https://doi.org/10.1021/ol501243g
Sharninghausen LS, Brooks AF, Winton WP, Makaravage KJ, Scott PJH, Sanford MS (2020) NHC-copper mediated ligand-directed radiofluorination of aryl halides. J Am Chem Soc 142:7362–7367. https://doi.org/10.1021/jacs.0c02637
Mossine AV, Brooks AF, Makaravage KJ, Miller JM, Ichiishi N, Sanford MS, Scott PJH (2015) Synthesis of [18F]arenes via the copper-mediated [18F]fluorination of boronic acids. Org Lett 17:5780–5783. https://doi.org/10.1021/acs.orglett.5b02875
Makaravage KJ, Brooks AF, Mossine AV, Sanford MS, Scott PJH (2016) Copper-mediated radiofluorination of arylstannanes with [18F]KF. Org Lett 18:5440–5443. https://doi.org/10.1021/acs.orglett.6b02911
Lee SJ, Makaravage KJ, Brooks AF, Scott PJH, Sanford MS (2019) Copper-mediated aminoquinoline-directed radiofluorination of aromatic C−H bonds with K18F. Angew Chem 131:3151–3154. https://doi.org/10.1002/ange.201812701
McCammant MS, Thompson S, Brooks AF, Krska SW, Scott PJH, Sanford MS (2017) Cu-mediated C-H 18F-fluorination of electron-rich (Hetero)arenes. Org Lett 19:3939–3942. https://doi.org/10.1021/acs.orglett.7b01902
Mossine AV, Brooks AF, Bernard-Gauthier V, Bailey JJ, Ichiishi N, Schirrmacher R, Sanford MS, Scott PJH (2018) Automated synthesis of PET radiotracers by copper-mediated 18F-fluorination of organoborons: importance of the order of addition and competing protodeborylation. J Label Compd Radiopharm 61:228–236. https://doi.org/10.1002/jlcr.3583
Jacobson O, Kiesewetter DO, Chen X (2015) Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug Chem 26:1–18. https://doi.org/10.1021/bc500475e
Block D, Coenen HH, Stacklin G (1988) 18F-fluoroalkylation of H-acidic compounds. J Label Compd Radiopharm 25:201–216. https://doi.org/10.1002/jlcr.2580250211
Kilbourn MR, Dence CS, Welch MJ, Mathias CJ (1987) Fluorine-18 labeling of proteins. J Nucl Med 28:462–471
Shai Y, Kirk KL, Channing MA, Dunn BB, Lesniak MA, Eastman RC, Finn RD, Roth J, Jacobson KA (1989) 18F-Labeled insulin: a prosthetic group methodology for incorporation of a positron emitter into peptides and proteins. Biochemistry 28:4801–4806. https://doi.org/10.1021/bi00437a042
Ivashkin P, Lemonnier G, Cousin J, Grégoire V, Labar D, Jubault P, Pannecoucke X (2014) CuCF3: A [18F]trifluoromethylating agent for arylboronic acids and aryl iodides. Chem A Eur J 20:9514–9518. https://doi.org/10.1002/chem.201403630
Rühl T, Rafique W, Lien VT, Riss PJ (2014) Cu(I)-mediated 18F-trifluoromethylation of arenes: rapid synthesis of 18F-labeled trifluoromethyl arenes. Chem Commun 50:6056–6059. https://doi.org/10.1039/C4CC01641F
Yang BY, Telu S, Haskali MB, Morse CL, Pike VW (2019) A gas phase route to [18F]fluoroform with limited molar activity dilution. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-50747-3
Vanderborn D, Sewing C, Herscheid JDMKDM, Windhorst AD, Orru RVAA, Vugts DJ, van der Born D, Sewing C, Herscheid JDMKDM, Windhorst AD, Orru RVAA, Vugts DJ (2014) A Universal procedure for the [18F]trifluoromethylation of aryl iodides and aryl boronic acids with highly improved specific activity. Angew Chem Int Ed 53:11046–11050. https://doi.org/10.1002/anie.201406221
Zheng J, Cheng R, Lin J-HH, Yu D-HH, Ma L, Jia L, Zhang L, Wang L, Xiao J-CC, Liang SH (2017) An unconventional mechanistic insight into SCF3 formation from difluorocarbene: preparation of 18F-labeled α-SCF3 carbonyl compounds. Angew Chem Int Ed 56:3196–3200. https://doi.org/10.1002/anie.201611761
Huiban M, Tredwell M, Mizuta S, Wan Z, Zhang X, Collier TL, Gouverneur V, Passchier J (2013) A broadly applicable [18F]trifluoromethylation of aryl and heteroaryl iodides for PET imaging. Nat Chem 5:941–944. https://doi.org/10.1038/nchem.1756
Kim HY, Lee JY, Lee YS, Jeong JM (2019) Design and synthesis of Enantiopure 18F-labelled [18F]trifluoromethyltryptophan from 2-halotryptophan derivatives via Copper(I)-mediated [18F]trifluoromethylation and evaluation of its in vitro characterization for the Ser. J Label Compd Radiopharm 62:566–579. https://doi.org/10.1002/jlcr.3772
Ponchant M, Hinnen F, Demphel S, Crouzel C (1997) [11C] Copper(I) cyanide : a new radioactive precursor for 11C-cyanation and functionalization of haloarenes. Appl Radiat Isot 48:755–762
Makaravage KJ, Shao X, Brooks AF, Yang L, Sanford MS, Scott PJH (2018) Copper(II)-mediated [11C]cyanation of arylboronic acids and arylstannanes. Org Lett 20:1530–1533. https://doi.org/10.1021/acs.orglett.8b00242
Rotstein BH, Hooker JM, Woo J, Collier TL, Brady TJ, Liang SH, Vasdev N (2014) Synthesis of [11C]bexarotene by Cu-mediated [11C]carbon dioxide fixation and preliminary PET imaging. ACS Med Chem Lett 5:668–672. https://doi.org/10.1021/ml500065q
Riss PJ, Lu S, Telu S, Aigbirhio FI, Pike VW (2012) CuI-catalyzed 11C carboxylation of boronic acid esters: a rapid and convenient entry to 11C-labeled carboxylic acids, esters, and amides. Angew Chem Int Ed 51:2698–2702. https://doi.org/10.1002/anie.201107263
Yang L, Brooks AF, Makaravage KJ, Zhang H, Sanford MS, Scott PJHH, Shao X (2018) Radiosynthesis of [11C]LY2795050 for preclinical and clinical PET imaging using Cu(II)-mediated cyanation. ACS Med Chem Lett 9:1274–1279. https://doi.org/10.1021/acsmedchemlett.8b00460
Matthews WB, Monn JA, Ravert HT, Holt DP, Schoepp DD, Dannals RF (2006) Synthesis of a mGluR5 antagonist using [11C]Copper(I) cyanide. J Label Compd Radiopharm 49:829–834. https://doi.org/10.1002/jlcr
Haskali MB, Pike VW (2017) [11C]Fluoroform, a breakthrough for versatile labeling of PET radiotracer trifluoromethyl groups in high molar activity. Chem A Eur J 23:8156–8160. https://doi.org/10.1002/chem.201701701
Ma L, Placzek MS, Hooker JM, Vasdev N, Liang SH (2017) Cyanation of arylboronic acids in aqueous solutions. Chem Commun 53:6597–6600. https://doi.org/10.1039/c7cc02886e
Zhou D, Chu W, Voller T, Katzenellenbogen JA (2018) Copper-mediated nucleophilic radiobromination of aryl boron precursors: convenient preparation of a radiobrominated PARP-1 inhibitor. Tetrahedron Lett 59:1963–1967. https://doi.org/10.1016/j.tetlet.2018.04.024
Ordonez AA, Carroll LS, Abhishek S, Mota F, Ruiz-Bedoya CA, Klunk MH, Singh AK, Freundlich JS, Mease RC, Jain SK (2019) Radiosynthesis and PET bioimaging of 76Br-bedaquiline in a murine model of tuberculosis. ACS Infect Dis 5:1996–2002. https://doi.org/10.1021/acsinfecdis.9b00207
Zhang P, Zhuang R, Guo Z, Su X, Chen X, Zhang X (2016) A highly efficient copper-mediated radioiodination approach using aryl boronic acids. Chem A Eur J 22:16783–16786. https://doi.org/10.1002/chem.201604105
Wilson TC, McSweeney G, Preshlock S, Verhoog S, Tredwell M, Cailly T, Gouverneur V (2016) Radiosynthesis of SPECT tracers: via a copper mediated 123I iodination of (hetero)aryl boron reagents. Chem Commun 52:13277–13280. https://doi.org/10.1039/c6cc07417k
Reilly SW, Makvandi M, Xu K, Mach RH (2018) Rapid Cu-catalyzed [211At]astatination and [125I]iodination of boronic esters at room temperature. Org Lett 20:1752–1755. https://doi.org/10.1021/acs.orglett.8b00232
Chun J-H, Lu S, Lee Y-S, Pike VW (2010) Fast and high-yield microreactor syntheses of ortho-substituted [18F]fluoroarenes from reactions of [18F]fluoride ion with diaryliodonium salts. J Org Chem 75:3332–3338. https://doi.org/10.1021/jo100361d
Goulding RW, Palmer AJ (1972) The preparation of fluorine-18 labelled p-fluorophenylalanine for clinical use. Int J Appl Radiat Isot 23:133–137. https://doi.org/10.1016/0020-708X(72)90085-3
Calabria F, Cascini GL (2015) Current status of 18F-DOPA PET imaging. Hell J Nucl Med 18:152–156
Nandu H, Wen PY, Huang RY (2018) Imaging in neuro-oncology. Ther Adv Neurol Disord 11:1–19. https://doi.org/10.1177/1756286418759865
Darcourt J, Schiazza A, Sapin N, Dufour M, Ouvrier M, Benisvy D, Fontana X, Koulibaly PM (2014) 18F-FDOPA PET for the diagnosis of parkinsonian syndromes. Q J Nucl Med Mol Imaging 58:355–365
Shah P, Demirbilek H, Hussain K (2014) Persistent hyperinsulinaemic hypoglycaemia in infancy. Semin Pediatr Surg 23:76–82. https://doi.org/10.1053/j.sempedsurg.2014.03.005
Richarz R, Krapf P, Zarrad F, Urusova EA, Neumaier B, Zlatopolskiy BD (2014) Neither azeotropic drying, nor base nor other additives: a minimalist approach to 18F-labeling. Org Biomol Chem 12:8094–8099. https://doi.org/10.1039/c4ob01336k
Zlatopolskiy BD, Zischler J, Krapf P, Zarrad F, Urusova EA, Kordys E, Endepols H, Neumaier B (2015) Copper-mediated aromatic radiofluorination revisited: efficient production of PET tracers on a preparative scale. Chem A Eur J 21:5972–5979. https://doi.org/10.1002/chem.201405586
Zischler J, Krapf P, Richarz R, Zlatopolskiy BD, Neumaier B (2016) Automated synthesis of 4-[18F]fluoroanisole, [18F]DAA1106 and 4-[18F]FPhe using Cu-mediated radiofluorination under “Minimalist” conditions. Appl Radiat Isot 115:133–137. https://doi.org/10.1016/j.apradiso.2016.04.030
Modemann DJ, Zlatopolskiy BD, Urusova EA, Zischler J, Craig A, Ermert J, Guliyev M, Endepols H, Neumaier B (2019) 2-[18F]Fluorophenylalanine: synthesis by nucleophilic 18F-fluorination and preliminary biological evaluation. Synthesis 51:664–676. https://doi.org/10.1055/s-0037-1611370
Yuan Z, Cheng R, Chen P, Liu G, Liang SH (2016) Efficient pathway for the preparation of aryl(isoquinoline)iodonium(III) salts and synthesis of radiofluorinated isoquinolines. Angew Chem Int Ed 55:11882–11886. https://doi.org/10.1002/anie.201606381
Yamaguchi A, Hanaoka H, Higuchi T, Tsushima Y (2018) Radiolabeled (4-fluoro-3-iodobenzyl)guanidine improves imaging and targeted radionuclide therapy of norepinephrine transporter-expressing tumors. J Nucl Med 59:815–821. https://doi.org/10.2967/jnumed.117.201525
Elie J, Vercouillie J, Arlicot N, Lemaire L, Bidault R, Bodard S, Hosselet C, Deloye JB, Chalon S, Emond P, Guilloteau D, Buron F, Routier S (2019) Design of selective COX-2 inhibitors in the (aza)indazole series. chemistry, in vitro studies, radiochemistry and evaluations in rats of a [18F] PET tracer. J Enzyme Inhib Med Chem 34:1–7. https://doi.org/10.1080/14756366.2018.1501043
Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF (2002) Mild Iridium-catalyzed borylation of arenes. high turnover numbers, room temperature reactions, and isolation of a potential intermediate. J Am Chem Soc 124:390–391. https://doi.org/10.1021/ja0173019
Cho J-Y, Tse MK, Holmes D, Maleczka RE, Smith MR (2002) Remarkably selective iridium catalysts for the elaboration of aromatic C–H Bonds. Science 295:305–308. https://doi.org/10.1126/science.1067074
Malapit CA, Bour JR, Laursen SR, Sanford MS (2019) Mechanism and scope of nickel-catalyzed decarbonylative borylation of carboxylic acid fluorides. J Am Chem Soc 141:17322–17330. https://doi.org/10.1021/jacs.9b08961
Wulff G, Lauer M (1983) Arylboronic acids with intramolecular B–N interaction: convenient synthesis through ortho-lithiation of subsituted benzylamines. J Organomet Chem 256:1–9
Kuehn L, Huang M, Radius U, Marder TB (2019) Copper-catalysed borylation of aryl chlorides. Org Biomol Chem 17:6601–6606. https://doi.org/10.1039/c9ob01244c
Wright JS, Scott PJH, Steel PG (2020) Iridium catalysed C-H borylation of heteroarenes: balancing steric and electronic regiocontrol. Angew Chem Int Ed. https://doi.org/10.1002/anie.202001520
Ishiyama T, Murata M, Miyaura N (1995) Palladium(0)-catalyzed cross-coupling reaction of alkoxydiboron with haloarenes: a direct procedure for arylboronic esters. J Org Chem 60:7508–7510. https://doi.org/10.1021/jo00128a024
Niwa T, Ochiai H, Watanabe Y, Hosoya T (2015) Ni/Cu-catalyzed defluoroborylation of fluoroarenes for diverse C–F bond functionalizations. J Am Chem Soc 137:14313–14318. https://doi.org/10.1021/jacs.5b10119
Teasdale A, Thompson S (2017) ICH Q3D elemental impurities. In: Teasdale A, Elder D, Nims RW (eds) ICH quality guidelines: an implementation guide. Wiley Ltd, Hoboken, pp 233–280. https://doi.org/10.1002/9781118971147
Taylor NJ, Emer E, Preshlock S, Schedler M, Tredwell M, Verhoog S, Mercier J, Genicot C, Gouverneur V (2017) Derisking the Cu-mediated 18F-fluorination of heterocyclic positron emission tomography radioligands. J Am Chem Soc 139:8267–8276. https://doi.org/10.1021/jacs.7b03131
Preshlock S, Calderwood S, Verhoog S, Tredwell M, Huiban M, Hienzsch A, Gruber S, Wilson TC, Taylor NJ, Cailly T, Schedler M, Collier TL, Passchier J, Smits R, Mollitor J, Hoepping A, Mueller M, Genicot C, Mercier J, Gouverneur V (2016) Enhanced copper-mediated 18F-fluorination of aryl boronic esters provides eight radiotracers for PET applications. Chem Commun 52:8361–8364. https://doi.org/10.1039/C6CC03295H
Zischler J, Kolks N, Modemann D, Neumaier B, Zlatopolskiy BD (2017) Alcohol-enhanced Cu-mediated radiofluorination. Chem A Eur J 23:3251–3256. https://doi.org/10.1002/chem.201604633
Zlatopolskiy BD, Zischler J, Schäfer D, Urusova EA, Guliyev M, Bannykh O, Endepols H, Neumaier B (2018) Discovery of 7-[18F]fluorotryptophan as a novel positron emission tomography (PET) probe for the visualization of tryptophan metabolism in vivo. J Med Chem 61:189–206. https://doi.org/10.1021/acs.jmedchem.7b01245
Mossine AV, Brooks AF, Ichiishi N, Makaravage KJ, Sanford MS, Scott PJH (2017) Development of customized [18F] fluoride elution techniques for the enhancement of copper-mediated late-stage radiofluorination. Sci Rep 7:233. https://doi.org/10.1038/s41598-017-00110-1
Antuganov D, Zykov M, Timofeeva K, Antuganova Y, Orlovskaya V, Krasikova R (2017) Effect of pyridine addition on the efficiency of copper-mediated radiofluorination of aryl pinacol boronates. ChemistrySelect 2:7909–7912. https://doi.org/10.1002/slct.201701628
Antuganov D, Zykov M, Timofeev V, Timofeeva K, Antuganova Y, Orlovskaya V, Fedorova O, Krasikova R (2019) Copper-mediated radiofluorination of aryl pinacolboronate esters: a straightforward protocol by using pyridinium sulfonates. Eur J Org Chem 2019:918–922. https://doi.org/10.1002/ejoc.201801514
Zhang X, Basuli F, Swenson RE (2019) An azeotropic drying-free approach for copper-mediated radiofluorination without addition of base. J Label Compd Radiopharm 62:139–145. https://doi.org/10.1002/jlcr.3705
Bernard-Gauthier V, Mossine AV, Mahringer A, Aliaga A, Bailey JJ, Shao X, Stauff J, Arteaga J, Sherman P, Grand’Maison M, Rochon P-LL, Wängler B, Wängler C, Bartenstein P, Kostikov A, Kaplan DR, Fricker G, Rosa-Neto P, Scott PJHH, Schirrmacher R, Grand’Maison M, Rochon P-LL, Wängler B, Wängler C, Bartenstein P, Kostikov A, Kaplan DR, Fricker G, Rosa-Neto P, Scott PJHH, Schirrmacher R (2018) Identification of [18F]TRACK, a fluorine-18-labeled tropomyosin receptor kinase (Trk) inhibitor for PET imaging. J Med Chem 61:1737–1743. https://doi.org/10.1021/acs.jmedchem.7b01607
Bailey JJ, Kaiser L, Lindner S, Wüst M, Thiel A, Soucy JP, Rosa-Neto P, Scott PJH, Unterrainer M, Kaplan DR, Wängler C, Wängler B, Bartenstein P, Bernard-Gauthier V, Schirrmacher R (2019) First-in-human brain imaging of [18F]TRACK, a PET tracer for tropomyosin receptor kinases. ACS Chem Neurosci 10:2697–2702. https://doi.org/10.1021/acschemneuro.9b00144
Giglio BC, Fei H, Wang M, Wang H, He L, Feng H, Wu Z, Lu H, Li Z (2017) Synthesis of 5-[18F]fluoro-alpha-methyl tryptophan: new trp based PET agents. Theranostics 7:1524–1530. https://doi.org/10.7150/thno.19371
Zhang Z, Lau J, Zhang C, Colpo N, Nocentini A, Supuran CT, Bénard F, Lin KS (2017) Design, synthesis and evaluation of 18f-labeled cationic carbonic anhydrase IX inhibitors for PET imaging. J Enzyme Inhib Med Chem 32:722–730. https://doi.org/10.1080/14756366.2017.1308928
Zhang Z, Lau J, Kuo HT, Zhang C, Colpo N, Bénard F, Lin KS (2017) Synthesis and evaluation of 18F-labeled CJ-042794 for imaging prostanoid EP4 receptor expression in cancer with positron emission tomography. Bioorganic Med Chem Lett 27:2094–2098. https://doi.org/10.1016/j.bmcl.2017.03.078
Litchfield M, Wuest M, Glubrecht D, Wuest F (2020) Radiosynthesis and biological evaluation of [18F]Triacoxib: a new radiotracer for PET imaging of COX-2. Mol Pharm 17:251–261. https://doi.org/10.1021/acs.molpharmaceut.9b00986
Wilson TC, Xavier MA, Knight J, Verhoog S, Torres JB, Mosley M, Hopkins SL, Wallington S, Allen PD, Kersemans V, Hueting R, Smart S, Gouverneur V, Cornelissen B (2019) PET imaging of PARP expression using 18F-olaparib. J Nucl Med 60:504–510. https://doi.org/10.2967/jnumed.118.213223
Guibbal F, Isenegger PG, Wilson TC, Pacelli A, Mahaut D, Sap JBI, Taylor NJ, Verhoog S, Preshlock S, Hueting R, Cornelissen B, Gouverneur V (2020) Manual and automated Cu-mediated radiosynthesis of the PARP inhibitor [18F]olaparib. Nat Protoc ASAP. https://doi.org/10.1038/s41596-020-0295-7
Clemente GS, Antunes I, Kurade S, Van WA, Dömling A, Elsinga PH (2019) Synthesis and preliminary preclinical evaluation of a 18F-fluorinated quaternary alpha-amino acid-based arginase inhibitor. J Label Compd Radiopharm 62(Suppl 1):S214
Haider A, Iten I, Ahmed H, Herde AM, Gruber S, Krämer SD, Keller C, Schibli R, Wünsch B, Mu L, Ametamey SM (2019) Identification and preclinical evaluation of a radiofluorinated benzazepine derivative for imaging the GluN2B subunit of the ionotropic NMDA receptor. J Nucl Med 60:259–266. https://doi.org/10.2967/jnumed.118.212134
Ahmed H, Haider A, Varisco J, Stanković M, Wallimann R, Gruber S, Iten I, Häne S, Müller Herde A, Keller C, Schibli R, Schepmann D, Mu L, Wünsch B, Ametamey SM (2019) Structure-affinity relationships of 2,3,4,5-tetrahydro-1 H-3-benzazepine and 6,7,8,9-tetrahydro-5 H-benzo[7]annulen-7-amine analogues and the discovery of a Radiofluorinated 2,3,4,5-tetrahydro-1 H-3-benzazepine congener for imaging GluN2B subunit-containing N-methyl-d-aspartate receptors. J Med Chem. https://doi.org/10.1021/acs.jmedchem.9b00812
Wu CY, Chen YY, Lin JJ, Li JP, Chen JK, Hsieh TC, Kao CH (2019) Development of a novel radioligand for imaging 18-kD translocator protein (TSPO) in a Rat Model of Parkinson’s Disease. BMC Med Imaging 19:1–9. https://doi.org/10.1186/s12880-019-0375-8
Pannell M, Economopoulos V, Wilson TC, Kersemans V, Isenegger PG, Larkin JR, Smart S, Gilchrist S, Gouverneur V, Sibson NR (2020) Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia. Glia 68:280–297. https://doi.org/10.1002/glia.23716
Keller T, Krzyczmonik A, Forsback S, Kirjavainen A, López-Picón FR, Takkinen JS, Dollé F, Rinne JO, Haaparanta-Solin M, Solin O (2017) Nucleophilic and electrophilic syntheses of [18F]F-DPA. J Label Compd Radiopharm 62(Suppl 1):S423
Keller T, López-Picón FR, Krzyczmonik A, Forsback S, Takkinen JS, Rajander J, Teperi S, Dollé F, Rinne JO, Haaparanta-Solin M, Solin O (2019) Comparison of high and low molar activity TSPO tracer [18F]F-DPA in a mouse model of Alzheimer’s Disease. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X19853117
Kaide S, Ono M, Watanabe H, Shimizu Y, Nakamoto Y, Togashi K, Yamaguchi A, Hanaoka H, Saji H (2018) Conversion of iodine to fluorine-18 based on iodinated chalcone and evaluation for β-amyloid PET imaging. Bioorganic Med Chem 26:3352–3358. https://doi.org/10.1016/j.bmc.2018.05.001
Li S, Cai Z, Wu X, Holden D, Pracitto R, Kapinos M, Gao H, Labaree D, Nabulsi N, Carson RE, Huang Y (2019) Synthesis and in vivo evaluation of a Novel PET radiotracer for imaging of synaptic vesicle glycoprotein 2A (SV2A) in nonhuman primates. ACS Chem Neurosci 10:1544–1554. https://doi.org/10.1021/acschemneuro.8b00526
Lai TH, Schroeder S, Ludwig F-A, Fischer S, Moldovan R, Scheunemann M, Dukic-Stefanovic S, Deuther-Conrad W, Steinbach J, Brust P (2019) Development of high affinity 18F-labelled radiotracers for PET imaging of the adenosine A2A receptor. J Label Compd Radiopharm 62(Suppl 1):S23
Lindberg A, Nag S, Schou M, Arakawa R, Nogami T, Moein MM, Elmore CS, Pike VW, Halldin C (2019) Development of a 18F-labeled PET Radioligand for Imaging 5-HT1B receptors: [18F]AZ10419096. Nucl Med Biol 78–79:11–16. https://doi.org/10.1016/j.nucmedbio.2019.10.003
Willmann M, Neumaier B, Ermert J (2019) A novel 18F-labeled D4-receptor ligand. J Label Compd Radiopharm 62(Suppl 1):S410–S411
Guibbal F, Meneyrol V, Ait-Arsa I, Diotel N, Patché J, Veeren B, Bénard S, Gimié F, Yong-Sang J, Khantalin I, Veerapen R, Jestin E, Meilhac O (2019) Synthesis and automated labeling of [18F]Darapladib, a Lp-PLA 2 Ligand, as potential PET imaging tool of atherosclerosis. ACS Med Chem Lett 10:743–748. https://doi.org/10.1021/acsmedchemlett.8b00643
Zhang Z, Zhang C, Lau J, Colpo N, Bénard F, Lin K-S (2016) One-step synthesis of 4-[18F]Fluorobenzyltriphenylphosphonium cation for imaging with positron emission tomography. J Label Compd Radiopharm 59:467–471. https://doi.org/10.1002/jlcr.3436
Bratteby K, Torkelsson E, L’Estrade ET, Peterson K, Shalgunov V, Xiong M, Leffler H, Zetterberg FR, Olsson TG, Gillings N, Nilsson UJ, Herth MM, Erlandsson M (2020) In vivo veritas: 18F-Radiolabeled glycomimetics allow insights into the pharmacological fate of galectin-3 inhibitors. J Med Chem. https://doi.org/10.1021/acs.jmedchem.9b01692
Attia K, Visser T, Steven J, Slart R, Antunes I, van der Hoek S, Elsinga PH, Heerspink H (2019) Synthesis and evaluation of [18F]Canagliflozin for imaging SGLT-2-transporters in diabetic patients. J Label Compd Radiopharm 62(Suppl 1):S27
Drerup C, Ermert J, Coenen HH (2016) Synthesis of a potent aminopyridine-based nNOS-inhibitor by two recent No-carrier-added 18F-labelling methods. Molecules. https://doi.org/10.3390/molecules21091160
Petersen IN, Kristensen JL, Herth MM (2017) Nucleophilic 18F-labeling of spirocyclic iodonium ylide or boronic pinacol ester precursors: advantages and disadvantages. Eur J Org Chem 2017:453–458. https://doi.org/10.1002/ejoc.201601448
Craig A, Kolks N, Urusova E, Zischler J, Neumaier B, Zlatopolskiy BD (2019) The efficient preparation of radiolabeled aromatic amino acids via cu-mediated radiofluorination of Ni-complexes. J Label Compd Radiopharm 62(Suppl 1):S118
Zhang X, Dunlow R, Blackman BN, Swenson RE (2018) Optimization of 18F-syntheses using 19F-reagents at tracer-level concentrations and liquid chromatography/tandem mass spectrometry analysis: improved synthesis of [18F]MDL100907. J Label Compd Radiopharm 61:427–437. https://doi.org/10.1002/jlcr.3606
Cole E, Donnelly D, Wallace M, Tran T, Burrell R, Turley W, Allentoff A, Huang A, Balog A, Bonacorsi S (2018) Radiochemistry challenges and progression for incorporation of 18F into a complex substituted 6–18F-fluoroquinoline BMS-986205 for IDO imaging. J Nucl Med 59(Suppl 1):605
Schäfer D, Zlatopolskiy BD, Ermert J, Neumaier B (2017) A practical two-step synthesis of 5-[18F]fluoro-l-tryptophan (5-[18F]FTrp) via alcohol-enhanced Cu-mediated radiofluorination. J Label Compd Radiopharm 60(Suppl 1):S105
Schäfer D, Weiß P, Ermert J, Castillo Meleán J, Zarrad F, Neumaier B (2016) Preparation of No-carrier-added 6-[18F]Fluoro-l-tryptophan via Cu-mediated radiofluorination. Eur J Org Chem 2016:4621–4628. https://doi.org/10.1002/ejoc.201600705
Milicevic Sephton S, Zhou X, Thompson S, Aigbirhio FI (2019) Preparation of the serotonin transporter PET radiotracer 2-({2-[(dimethylamino)methyl]phenyl}thio)-5-[18F]fluoroaniline (4-[18F]ADAM): probing synthetic and radiosynthetic methods. Synthesis 51:4374–4384. https://doi.org/10.1055/s-0039-1690522
Clemente GS, Zarganes-tzitzikas T, Dömling A, Elsinga PH (2019) Late-stage copper-catalyzed radiofluorination of an arylboronic ester derivative of atorvastatin. Molecules 24:4210–4218. https://doi.org/10.3390/molecules24234210
Mossine AV, Tanzey SS, Brooks AF, Makaravage KJ, Ichiishi N, Miller JM, Henderson BD, Skaddan MB, Sanford MS, Scott PJH (2019) One-pot synthesis of high molar activity 6-[18F]fluoro-l-DOPA by Cu-mediated fluorination of a Bpin precursor. Org Biomol Chem 17:8701–8705. https://doi.org/10.1039/c9ob01758e
Blevins DW, Kabalka GW, Osborne DR, Akula MR (2018) Effect of added Cu(OTf)2 on the Cu(OTf)2(Py)4-mediated radiofluorination of benzoyl and phthaloylglycinates. Nat Sci 10:125–133. https://doi.org/10.4236/ns.2018.103013
Blevins DW, Akula MR, Kabalka GW, Osborne DR (2017) Synthesis of 4(4-[18F]Fluorophenyl)piracetam, a potential PET agent for Parkinson’s Disease. J Label Compd Radiopharm 62(Suppl 1):S171. https://doi.org/10.1002/jlcr.3725
Honda N, Yoshimoto M, Mizukawa Y, Katsuhiko O, Kanai Y, Kurihara H, Tateishi H, Takahashi K (2017) Radiosynthesis of 2-[18F]fluoro-4-boronophenylalanine ([18F]FBPA using copper mediated oxidative aromatic nucleophilic [18F]Fluorination. J Label Compd Radiopharm 60(Suppl 1):S512. https://doi.org/10.1002/jlcr.3508
Ludwig F-A, Fischer S, Moldovan R, Deuther-Conrad W, Kranz M, Schepmann D, Jia H, Wünsch B, Brust P (2019) Development of a New 18F-labeled radioligand for imaging Sigma2 receptors by positron emission tomography. J Label Compd Radiopharm 62(Suppl 1):S181–S182
Zarganes-Tzitzikas T, Clemente GS, Elsinga PH, Dömling A (2019) MCR Scaffolds get Hotter with 18F-labeling. Molecules 24:1–15. https://doi.org/10.3390/molecules24071327
Gribanov PS, Golenko YD, Topchiy MA, Minaeva LI, Asachenko AF, Nechaev MS (2018) Stannylation of aryl halides, stille cross-coupling, and one-pot, two-step stannylation/stille cross-coupling reactions under solvent-free conditions. Eur J Org Chem 2018:120–125. https://doi.org/10.1002/ejoc.201701463
Gilman H, Rosenberg SD (1953) Reaction of triphenyltin-lithium with organic halides. J Org Chem 18:680–685. https://doi.org/10.1021/jo01134a012
Furuya T, Strom AE, Ritter T (2009) Silver-mediated fluorination of functionalized aryl stannanes. J Am Chem Soc 131:1662–1663. https://doi.org/10.1021/ja8086664
Teare H, Robins EG, Kirjavainen A, Forsback S, Sandford G, Solin O, Luthra SK, Gouverneur V (2010) Radiosynthesis and evaluation of [18F]selectfluor bis(triflate). Angew Chemie - Int Ed 49:6821–6824. https://doi.org/10.1002/anie.201002310
Adam MJ, Pate BD, Ruth TJ, Berry JM, Hall LD (1981) Cleavage of Aryl-Tin bonds with elemental fluorine: rapid synthesis of [18F]Fluorobenzene. J Chem Soc Chem Commun. https://doi.org/10.1039/C39810000733
Eskola O, Grönroos T, Bergman J, Haaparanta M, Marjamäki P, Lehikoinen P, Forsback S, Langer O, Hinnen F, Dollé F, Halldin C, Solin O (2004) A novel electrophilic synthesis and evaluation of medium specific radioactivity (1R,2S)-4-[18F]fluorometaraminol, a tracer for the assessment of cardiac sympathetic nerve integrity with PET. Nucl Med Biol 31:103–110. https://doi.org/10.1016/S0969-8051(03)00098-2
Ye Y, Sanford MS (2013) Mild copper-mediated fluorination of aryl stannanes and aryl trifluoroborates. J Am Chem Soc 135:9. https://doi.org/10.1021/ja400300g
Alvarez M, Le Bars D (2012) Synthesis of 4‐(2′‐methoxyphenyl)‐1‐[2′‐(N‐2″Pyridinyl)‐p‐[18F]fluorobenzamido]ethylpiperazine [18F]MPPF. In Scott PJH, Hockley BG (eds) Radiochemical syntheses, vol 1. Wiley, Hoboken, pp 87−94. https://doi.org/10.1002/9781118140345.ch10
Bowden GD, Franke A, Pichler BJ, Maurer A (2019) Automated synthesis of [18F]O6-[(4-[18F]fluoro)benzyl]guanine ([18F]pFBG) via [18F]-fluorobenzyl alcohol ([18F]4FBnOH) from an optimized copper mediated radiofluorination (CMRF). J Label Compd Radiopharm 62(Suppl 1):S329–330
Bowden GD, Pichler BJ, Maurer A (2019) A design of experiments (DoE) approach accelerates the optimization of copper-mediated 18F-fluorination reactions of arylstannanes. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-47846-6
Zarrad F, Zlatopolskiy B, Krapf P, Zischler J, Neumaier B (2017) A practical method for the preparation of 18F-labeled aromatic amino acids from nucleophilic [18F]fluoride and stannyl precursors for electrophilic radiohalogenation. Molecules 22:2231. https://doi.org/10.3390/molecules22122231
Lahdenpohja S, Keller T, Rajander J, Kirjavainen AK (2019) Radiosynthesis of the norepinephrine transporter tracer [18F]NS12137 via copper-mediated 18F-labelling. J Label Compd Radiopharm 62:259–264. https://doi.org/10.1002/jlcr.3717
Lahdenpohja SO, Rajala NA, Rajander J, Kirjavainen AK (2019) Fast and efficient copper-mediated 18F-fluorination of arylstannanes, aryl boronic acids, and aryl boronic esters without azeotropic drying. EJNMMI Radiopharm Chem. https://doi.org/10.1186/s41181-019-0079-y
Onwordi EC, Halff EF, Whitehurst T, Mansur A, Cotel MC, Wells L, Creeney H, Bonsall D, Rogdaki M, Shatalina E, Reis Marques T, Rabiner EA, Gunn RN, Natesan S, Vernon AC, Howes OD (2020) Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in Rats. Nat Commun. https://doi.org/10.1038/s41467-019-14122-0
Li S, Naganawa M, Zheng M, Pracitto R, Henry S, Matuskey D, Kapinos M, Emery P, Cai Z, Ropchan J, Labaree D, Nabulsi N, Carson R, Huang Y (2019) First-in-human evaluation of 18F-SDM-8, a novel radiotracer for PET imaging of synaptic vesicle glycoprotein 2A. J Nucl Med 60:49
Constantinescu CC, Tresse C, Zheng MQ, Gouasmat A, Carroll VM, Mistico L, Alagille D, Sandiego CM, Papin C, Marek K, Seibyl JP, Tamagnan GD, Barret O (2019) Development and in vivo preclinical imaging of fluorine-18-labeled synaptic vesicle protein 2A (SV2A) PET tracers. Mol Imaging Biol 21:509–518. https://doi.org/10.1007/s11307-018-1260-5
Blevins DW, Akula MR, Kabalka GW, Osborne DR (2019) An improved synthesis of 4-(4-[18F]fluorophenyl)pircetam a PET agent for Parkinson’s disease. J Label Compd Radiopharm 62(Suppl 1):S171
Yao B, Wang ZL, Zhang H, Wang DX, Zhao L, Wang MX (2012) Cu(ClO4)2-mediated arene C–H Bond halogenations of azacalixaromatics using alkali metal halides as Halogen sources. J Org Chem 77:3336–3340. https://doi.org/10.1021/jo300152u
Yao B, Wang DX, Huang ZT, Wang MX (2009) Room temperature aerobic formation of a stable aryl-Cu(III) complex and its reactions with nucleophiles: highly efficient and diverse arene C–H functionalizations of azacalix[1]arene[3]pyridine. Chem Commun. https://doi.org/10.1039/b902946j
Zhang H, Yao B, Zhao L, Wang DX, Xu BQ, Wang MX (2014) Direct synthesis of high-valent aryl-Cu(II) and Aryl-Cu(III) compounds: mechanistic insight into arene C–H bond metalation. J Am Chem Soc 136:6326–6332. https://doi.org/10.1021/ja412615h
Truong T, Klimovica K, Daugulis O (2013) Copper-catalyzed, directing group-assisted fluorination of arene and heteroarene C–H bonds. J Am Chem Soc 135:9342–9345. https://doi.org/10.1021/ja4047125
Mossine A, Tanzey S, Brooks A, Makaravage K, Ichiishi N, Miller J, Henderson B, Erhard T, Bruetting C, Skaddan M, Sanford M, Scott P (2020) Synthesis of high molar activity [18F]6-fluoro-l-DOPA suitable for human use by Cu-mediated fluorination of a BPin precursor. Nat Protoc. https://doi.org/10.1038/s41596-020-0305-9
Funding
Funding from the National Institutes of Health (R01EB021155 to P.J.H.S and M.S.S; F32GM136022 to L.S.S) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
All authors participated in literature search, literature review, manuscript writing, manuscript editing and content planning.
Corresponding authors
Ethics declarations
Conflict of interest
All authors (Jay S. Wright, Tanpreet Kaur, Sean Preshlock, Sean S. Tanzey, Wade P. Winton, Liam S. Sharninghausen, Nicholas Wiesner, Allen F. Brooks, Melanie S. Sanford and Peter J. H. Scott) declare that there is no conflict of interest regarding the publication of this article.
Ethical approval
This review article does not contain any original studies with human or animal subjects performed by any of the authors.
Additional information
This article is dedicated to the memory of Dr. Giovanni Lucignani, Editor-in-Chief of Clinical and Translational Imaging, who sadly passed away recently. The energy and enthusiasm he brought to this field will be greatly missed. Ciao, amico mio.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wright, J.S., Kaur, T., Preshlock, S. et al. Copper-mediated late-stage radiofluorination: five years of impact on preclinical and clinical PET imaging. Clin Transl Imaging 8, 167–206 (2020). https://doi.org/10.1007/s40336-020-00368-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-020-00368-y