Abstract
Purpose
Despite the excellent capacity of the conventional MRI to image brain tumours, problems remain in answering a number of critical diagnostic questions. To overcome these diagnostic shortcomings, PET using radiolabeled amino acids and perfusion-weighted imaging (PWI) are currently under clinical evaluation. The role of amino acid PET and PWI in different diagnostic challenges in brain tumours is controversial.
Methods
Based on the literature and experience of our centres in correlative imaging with PWI and PET using O-(2-[18F]fluoroethyl)-l-tyrosine or 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine, the current role and shortcomings of amino acid PET and PWI in different diagnostic challenges in brain tumours are reviewed. Literature searches were performed on PubMed, and additional literature was retrieved from the reference lists of identified articles. In particular, all studies in which amino acid PET was directly compared with PWI were included.
Results
PWI is more readily available, but requires substantial expertise and is more sensitive to artifacts than amino acid PET. At initial diagnosis, PWI and amino acid PET can help to define a site for biopsy but amino acid PET appears to be more powerful to define the tumor extent. Both methods are helpful to differentiate progression or recurrence from unspecific posttherapeutic changes. Assessment of therapeutic efficacy can be achieved especially with amino acid PET, while the data with PWI are sparse.
Conclusion
Both PWI and amino acid PET add valuable diagnostic information to the conventional MRI in the assessment of patients with brain tumours, but further studies are necessary to explore the complementary nature of these two methods.
Similar content being viewed by others
Introduction
Cerebral gliomas arising from different brain tissue types are the most prevalent primary brain tumours with an incidence of 5–6 in 100,000, apart from meningiomas [1]. Metastases in the brain originating from various peripheral tumours are even more frequent tumours with an incidence of 8−14/100.000 [2]. Histologically, gliomas are subdivided into astrocytomas, oligodendrogliomas, ependymal tumours, and tumours of the choroids plexus. The classification of gliomas by the World Health Organization (WHO) has been updated recently, combining now molecular parameters, such as IDH mutation and 1p/19q co-deletion with histology [3].
During the diagnostic process of brain lesions, it may be crucial to differentiate brain tumours from benign lesions, such as demyelination, hematoma, abscesses, and infarctions which may appear similar on MRI. MRI is at present the standard neuroimaging modality [4] owing to its excellent soft-tissue contrast and spatial resolution. The standard MRI for diagnostic imaging in brain tumours is based on pre- and postcontrast T1-weighted images and T2-weighted images, including fluid-attenuated inversion recovery (FLAIR) images. However, there are limitations in the standard MRI with regard to differentiating tumour tissue from nonspecific changes, which is especially relevant after therapy.
With positron emission tomography (PET), different radioactively labelled tracers are injected to target various metabolic and molecular pathways. This may add important information especially in clinically challenging situations to improve diagnosis and therapy planning. Over the past decades, PET with radiolabelled amino acids has become a highly relevant diagnostic tool. Recent joint recommendations of the Response Assessment in Neuro-Oncology working group (RANO) and the European Association for Neuro-Oncology (EANO) consider amino acid PET as clinically helpful and suggest its use for managing patients with brain tumours additionally to MRI [5]. Meanwhile, advanced MRI methods, such as perfusion-weighted imaging (PWI), are being evaluated in the clinical setting and can provide complementary pathophysiological information to the standard MRI. Based on the experience of our centres in correlative imaging with PWI and PET using O-(2-[18F]fluoroethyl)-l-tyrosine (FET) or 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (FDOPA) in more than 500 brain tumour patients, this review focuses on the clinical impact of amino acid PET and PWI in adult patients during the workup of brain tumours. Literature searches were performed on PubMed using the search terms “brain tumours”, “gliomas” “positron emission tomography”, “magnetic resonance imaging”, “Amino acids”, “methionine”, “FET, “FDOPA”, “perfusion imaging”, “PET”, and “rCBV”. Additional literature was retrieved from the reference lists of identified articles. Only papers in the English language published until the end of 2016 were selected for review. The references cited in the review were selected by the authors with respect to the scientific quality, with preference to more recent publications, and relevance of the papers in the field according to the personal experience of the authors. In particular, all studies were included in which amino acid PET was directly compared with PWI. These studies are summarized in Table 1. The performance of amino acid PET and PWI is discussed with respect to differential diagnosis of brain lesions, tumor delineation and biopsy guidance as well as therapy monitoring and discrimination between tumour progression or recurrence and treatment-related changes.
PET using radiolabelled amino acids
Amino acids present several advantages over 18F-2-fluoro-2-deoxy-d-glucose (FDG) and are now regarded as the tracers of choice for PET imaging of brain tumours [6]. The first radiopharmaceutical of this class to be used clinically was 11C-methyl-l-methionine (MET), an isotopically labelled methionine made from 11CH3-I alkylation of homocysteine [7]. Data on the clinical usefulness of MET in brain tumours have been collected for more than 30 years; however, due to the short half-life of 11C (20 min), which limits the use of MET to centres equipped with an onsite cyclotron, the interest around fluorinated compounds has grown significantly in the recent past. The two most popular amino acid PET radiopharmaceuticals labelled with 18F (109.8 min half-life) are O-(2-[18F]fluoroethyl)-l-tyrosine (FET) and 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (FDOPA). FET is a tyrosine derivative, which is synthesized with high yield by a two-step fluoroethylation of tyrosine [8]. FDOPA is an analogue of the non-proteinogenic amino acid l-DOPA, which has historically been synthesized via electrophilic substitution. However, because of isomeric impurities, low specific activity, low radiochemical yield, and precursor toxicity, this process has been later abandoned for nucleophilic substitution [9, 10]. The molecular structures of the abovementioned synthetic amino acids are shown in Fig. 1.
With the exception of MET, these radiolabelled amino acids are not incorporated into proteins [11]; nevertheless, their uptake mechanisms are highly efficient, leading to very favourable tumour-to-background ratios. The uptake of radiolabelled amino acids is based on the expression of the Na+-independent large neutral amino acid transporters on the cell surface of tumour cells, namely, LAT1 and LAT2. This mechanism is independent from blood–brain barrier permeability; therefore, amino acid probes are able to depict non contrast-enhancing brain tumour regions, which are a clear advantage over other PET tracers, such as 3′-deoxy-3′-[18F]fluorothymidine (FLT) [12] and 18F-Fluorocholine (FCH) [13]. MET and FDOPA uptakes are thought to be largely due to LAT1 [14, 15], while FET is transported by both LAT1 and LAT2 [16]. A recent study showed that the retention of FET into cells is due to an asymmetric intra- and extracellular recognition of LAT1, with consequent poor efflux from the cell back to the extracellular space [17].
No relevant differences between available radiolabelled amino acids have been shown in terms of tumor to brain contrast [18–21]. However, some differences exist with regard to tracer biodistribution in the brain and the time-activity curves of tracer uptake. FDOPA is a substrate for the enzyme aromatic amino acid decarboxylase in dopaminergic neurons [22]; this is responsible for FDOPA prominent uptake by the basal ganglia which might interfere with tumour delineation [23]. In addition, available data suggest that FET kinetics may add additional biological information, which may be helpful for glioma grading [24, 25], the differentiation of both glioma and brain metastasis recurrence from radiation-induced changes [26–28] or the prognostication of untreated gliomas [29, 30]. Although amino acid PET shows high accuracy for the detection of brain tumours [31], tracer uptake in non-neoplastic brain lesions has to be taken into account. Thus, unspecific amino acid uptake in the brain has been reported in multiple sclerosis and other inflammatory lesions, vascular malformations, ischemic lesions, hematomas, etc [32–36]. Moreover, amino acid PET may be negative in a significant proportion of low-grade gliomas [37, 38].
MRI and MR perfusion imaging
MRI is the method of choice for anatomical imaging in patients suspicious for brain tumours as well as during the course of therapy in brain tumour patients due to its excellent soft-tissue characterization, high resolution, and easy multiplanar reconstruction. The standard MRI includes pre- and postcontrast T1-weighted sequences as well as T2-weighted sequences possibly with FLAIR sequences [4, 39–41]. MRI has a high sensitivity to detect brain lesions due to its excellent soft-tissue contrast, but hyperintense signal in T2-weighted images may also be caused by peritumoural oedema. In tumours with diffuse cell infiltration, a clear demarcation of tumour from peritumoural changes may not be possible. In contrast, enhancement in T1-weighted images indicates a disruption of the blood–brain barrier (BBB) and is typical but not specific for malignant tumours. Particularly, treatment-related changes after surgery, radio-, or chemotherapy of brain tumours may also cause contrast enhancement and be challenging to differentiate from tumour recurrence [4, 42, 43].
PWI is an advanced MRI method which provides information on brain hemodynamics in addition to the conventional MRI [44, 45]. Important parameters which can be determined by PWI are the relative cerebral blood volume (rCBV), relative cerebral blood flow (rCBF), and the relative mean transit time (MTT) [45–47]. PWI is usually performed with paramagnetic gadolinium as an exogenous contrast agent which causes an apparent signal increase in T1-weighted images and a signal loss in T2 or T2*-weighted images. Dynamic susceptibility contrast MRI (DSC) based on T2*weighted imaging using a 2D echo planar imaging (EPI) is the most widely used method for PWI [44, 45]. Blood–brain barrier leakage may be a source of error with DSC which can be reduced by a pre-bolus of contrast agent prior to acquisition as well as post-processing techniques for leakage correction [45, 48]. Another method, dynamic contrast-enhanced MRI (DCE) is also based on a short bolus of contrast agent and measures changes in T1-weighted images over time. DCE is less prone to artifacts, but signal changes are smaller compared with DSC which results in rather low signal-to-noise ratio in the calculated parametric maps. Therefore, DCE is infrequently used in clinical practice and not included in the standard software of most scanners [47]. Recently, however, multi-echo sequences have been proposed to correct for leakage effect for DSC [49] and to extract T1 dynamics [50]. By combining DSC and DCE in a GRE and SE sequence (GESE) [51], further information about vasculature can be obtained [52]. Such parametric images can also be used to track therapy response in brain tumours to antiangiogenic agents [53]. Moreover, CBF can be measured by arterial spin labeling (ASL) using magnetically labelled water as endogenous tracer which avoids an exogenous contrast agent but the long acquisition time is a disadvantage [45].
Amino acid PET and MR perfusion imaging for tumour differential diagnosis
If a brain lesion of unclear origin is detected in a patient, it is of paramount clinical importance to differentiate between a neoplastic or non-neoplastic process. MRI with gadolinium-based contrast agents provides a number of imaging features, which may allow a differential diagnosis in a considerable fraction of the lesions. The imaging findings of brain tumours may include diffusely delineated tumour margins, perifocal oedema, central necrosis, presence of cystic formations, and a ring-enhancing pattern of contrast enhancement. The reliability of these signs, however, is limited. PWI can be helpful in special situations, for example, to differentiate brain abscesses from malignant gliomas or brain metastases [54].
PET using radiolabelled amino acids generally shows higher uptake in neoplastic lesions than in non-neoplastic lesions and increased amino acid uptake in benign lesions is rare. Nevertheless, false-positive uptake of MET, FET and FDOPA has been reported in infectious lesions (e.g., in brain abscesses), demyelinating lesions, ischemic stroke, in cerebral haemorrhages, and also in patients with status epilepticus or seizure clusters [34, 55]. Regarding differential diagnosis, a meta-analysis evaluated 462 patients with newly diagnosed brain lesions suspected of being brain tumours and revealed a pooled sensitivity of 82% and specificity of 76% for the correct diagnosis of primary brain tumours [56] based on a threshold of the maximum tumour/brain ratio of 2.1. A subsequent study evaluated 174 patients with newly diagnosed brain lesions and reported a high specificity (92%), but a lower sensitivity (57%) for the differentiation of neoplastic from non-neoplastic tissue [35]. Nevertheless, in that study, a maximum tumour/brain ratio of 2.5 or more yielded a very high positive predictive value for neoplastic tissue of 98%. Comparative studies between amino acid PET and PWI in this field are not yet available in the literature.
In summary, imaging findings from both the conventional MRI and PWI as well as amino acid PET may add valuable additional information for the characterization of equivocal brain lesions, but they lack sufficient diagnostic accuracy. Therefore, a histological evaluation of these suspicious brain lesions by biopsy or resection is frequently indispensable.
Amino acid PET and MR perfusion imaging for imaging of tumour extent and biopsy guidance
The diagnostic performance of the conventional MRI to depict the true extent of cerebral gliomas and to detect the most aggressive areas in inhomogeneous gliomas is limited and especially difficult in gliomas showing no contrast enhancement. Representative tissue samples of brain tumours are vitally important for the correct histological tumour diagnosis and grading, evaluation of molecular markers (e.g., IDH mutation), prognostication, and treatment decisions. Particularly, in infiltrating brain tumours with enhancing and non-enhancing tumour portions, the correct delineation of tumour extent and the identification of the most malignant parts can be challenging and may result in under-diagnosis.
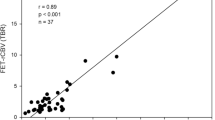
For PET, a number of studies have explored the spatial correlation of histopathological findings with amino acid uptake and provided evidence that the solid tumour mass of gliomas typically shows increased amino acid uptake and detects tumour extent more reliably than standard MRI [57–62] (Fig. 2). The improved delineation of tumor extent is one of the most important advances in brain tumor diagnostics provided by amino acid PET, but it has to be considered that a fraction of approx. 5% of all gliomas, especially low-grade gliomas, do not accumulate radiolabeled amino acids [35]. Nevertheless, amino acid PET is clearly superior to the standard MRI and the property of amino acid PET to detect tumour extent has been used in many studies for treatment planning, especially in tumor resection and radiation therapy [63–73]. In contrast, only a few studies have investigated the diagnostic value of PWI for the detection of brain tumour extent [74, 75]. Those studies observed elevated rCBV beyond the contrast-enhancing volume indicating that tumour infiltration might also be detected by PWI. A biopsy-controlled study reported that rCBV regionally correlates with both cell and microvessel density within gliomas [76]. Another biopsy-controlled study demonstrated that rCBV correlated with cell proliferation in high-grade gliomas but a correlation of rCBV with tumour cell density could not be confirmed [74]. These studies suggest that rCBV mapping allows only very limited conclusions with regard to tumor extension. While some earlier studies comparing amino acid uptake and rCBV observed similarities between MET uptake and rCBV abnormalities in gliomas [77, 78], more recent publications reported on considerably larger tumour volumes in amino acid PET than in rCBV maps and a poor spatial overlap [23, 79–82] (Figs. 3, 4; Table 1). Furthermore, rCBV mapping exhibited a lower lesion-to-brain contrast and a highly variable background noise as compared with amino acid PET [23, 79]. Another hybrid PET/MRI study reported that artifacts due to susceptibility differences between bone and air, iron accumulations, and blood degradation products hampered interpretation of the rCBV signal in the tumour area in 56% of the patients [80]. Thus, amino acid PET appears to be superior to rCBV mapping for the detection of the extent of cerebral gliomas and interpretation of rCBV maps appears to be more challenging than with amino acid PET.
Comparison of MRI and FET PET of patient with an anaplastic astrocytoma WHO grade III. Contrast-enhanced T1-weighted MRI (A) shows pathological contrast enhancement in the vicinity of the posterior horn of the right ventricle and corresponding signal abnormalities in the T2-weighted image (B). FET PET (C) detects metabolically active tumor tissue extending beyond the abnormalities in MRI
Hybrid PET/MRI of patient with an astrocytoma WHO grade II. Contrast-enhanced T1-weighted MR imaging (A) shows a small area with contrast enhancement in the left frontal lobe while FET PET (C) detects a large tumor extending within the area of signal abnormality in the FLAIR image (B). Tumor depiction in rCBV map (D) is difficult because of a poor tumor to brain contrast
Comparison between MRI and 18F-DOPA PET of a patient with an astrocytoma. A non-enhancing (A), FLAIR positive (B) left temporo-thalamic lesion is seen, corresponding to 18F-DOPA uptake (C) above the physiological radioactivity of the basal ganglia. In contrast, rCBV map (D) fails to show increased tumor perfusion. An anaplastic transformation was observed 3 months later, characterized by contrast enhancement and increased rCBV (images not shown)
Regarding biopsy guidance, parametric rCBV maps have been used to target the most malignant tumour parts, since the rCBV may indicate tumour parts with neovascularization. Some studies directly comparing local hot spots in amino acid PET and rCBV maps in gliomas reported on a spatial colocalization of local maxima [77, 83], but other studies observed considerable spatial distances of the local maxima in both methods [23, 79] (Table 1; Figs. 5, 6). It remains unclear whether the spatial position of the local maxima of amino acid uptake or rCBV in gliomas correspond to the most aggressive part of the tumour and further comparative studies are needed to investigate this aspect. Amino acid has been shown to be a very sensitive method to identify metabolic hot spots in gliomas for biopsy guidance [84–86]. In low-grade gliomas, a sensitivity of 72–79% has been reported for FET PET to identify a hot spot for biopsy guidance [35, 84]. Furthermore, the evaluation of kinetic parameters such as time-to-peak values or the curve pattern of FET uptake derived from dynamic PET scans in gliomas appears to be helpful to identify areas of malignant progression and unfavourable prognosis [29, 30, 37, 87–89]. These data highlight the potential of amino acid PET for the identification of metabolically active areas in brain tumours to target biopsies.
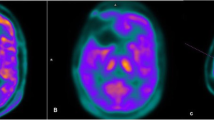
Hybrid PET/MRI of patient with a glioblastoma WHO grade IV. Contrast-enhanced T1-weighted MR imaging (A) shows a small area with contrast enhancement in right parietal lobe and corresponding signal abnormality in the FLAIR image (B) which shows focal tracer uptake in FET PET (C) and correspondingly increased rCBV (D)
Hybrid PET/MRI of a patient with an oligoastrocytoma WHO grade III. Contrast-enhanced T1-weighted MR imaging (A) shows a large mass in the right parietal lobe showing no contrast enhancement. FET PET (B) detects a local maximum in the paramedian part of the tumour which is not obvious in the rCBV map (C)
In summary, both amino acid PET and PWI may be helpful for biopsy guidance, but the more reliable method to delineate the glioma extent seems to be amino acid PET.
Amino acid PET and MR perfusion imaging for tumour grading and prognosis
The evaluation of brain tumor histology is the method of choice for tumour grading and decisive for treatment planning. Tissue samples, however, are sometimes not representative of the most aggressive tumor parts and varying interpretation by different neuropathologists may cause uncertainties. Therefore, an agreement of histopathology with non-invasive imaging parameters may support the clinical decision-making.
Most studies using amino acid PET in gliomas come to the conclusion that amino acid uptake does not allow a reliable prediction of tumour grade owing to highly variable amino acid uptake in gliomas of different WHO grades [90]. Glioma grading is further affected by high amino acid uptake in oligodendrogliomas despite the better prognosis of these tumours in comparison with astrocytomas of the same grade [91–94]. Therefore, static amino acid PET achieves only an accuracy of 70–80% for predicting a high-grade gliomas [31, 35, 93, 95]. Analysis of FET kinetics may slightly improve the discrimination of high-grade and low-grade glioma in both primary tumours and recurrent tumours [24, 25, 29, 88, 93, 96, 97].
The significance of PWI for tumour grading has been investigated in many studies with variable results [54, 98–101]. One study reported no difference between PWI and contrast-enhanced MRI [102]. Especially, low-grade oligodendroglioma may exhibit increased rCBV despite an excellent prognosis similar to the findings with amino acid PET [103]. A prospective study with 129 patients achieved a high accuracy in tumour grading with the conventional MRI based on the parameters contrast enhancement and necrosis (sensitivity 96%; specificity 70%) and the results did not improve when including PWI [104]. In contrast, a recent meta-analysis came to the conclusion that differentiation of low- and high-grade gliomas was improved by PWI compared with the conventional MRI [105].
While the prognostic significance of amino acid uptake ratios in gliomas remains questionable, recent studies indicate that the “biological tumour volume” (BTV) as determined by amino acid PET represents an independent prognostic factor [106–108]. In addition, amino acid PET seems to be useful to predict survival in patients with low-grade gliomas [38, 109–113]. In patients with newly diagnosed low-grade glioma, FET PET together with anatomical MR has been reported to be a significant factor to predict outcome [38]. In low-grade gliomas, particularly, FET kinetics may be useful to locate regions of malignant transformation and poor prognosis [30, 37, 87, 89, 114]. Using PWI, some studies have reported a relationship between rCBV in gliomas and overall survival [115–117]. A first study comparing FDOPA PET and rCBV in a small cohort of low-grade gliomas showed a better correlation of PET parameters with outcome than rCBV [118].
In summary, amino acid PET and PWI can support non-invasive grading and prediction of outcome in gliomas. However, the final diagnosis is based on histology of tumour tissue.
Amino acid PET and MR perfusion imaging for the diagnosis of tumour recurrence/progression
The distinction between tumour recurrence or progression and treatment-related changes represents a major challenge for the conventional MRI, since subacute and late types of treatment-induced injury, namely, pseudoprogression and radionecrosis, are characterized by an increase of contrast enhancement, indistinguishable from tumor progression [119]. Pseudoprogression usually occurs within the first 12 weeks after irradiation in 10–30% of patients with high-grade gliomas treated with concomitant temozolomide [120]. A recent study using FET PET showed an excellent overall accuracy of 96% (sensitivity, 100%; specificity, 91%) in discriminating pseudoprogression from early progression in 22 patients with glioblastoma [26]. Studies assessing pseudoprogression with perfusion-weighted MRI are more numerous though less promising, probably because rCBV is influenced by disruption of the BBB [121–125]. A recent meta-analysis yielded a pooled sensitivity of 89% and a pooled specificity of 80% for DSC perfusion-weighted imaging in this clinical setting [126]. Diagnostic accuracy of DCE perfusion-weighted images has been reported in the same range of values [127].
More data are available on the performances of amino acid PET to differentiate tumor recurrence from treatment-related changes in the later stage of the disease. Earlier experiences with FET showed the superiority of amino acid PET over the standard MRI in this setting [128]. In a large heterogeneous cohort of 124 patients with gliomas of different grades and histologies, Galldiks and coworkers found an accuracy of 93% (sensitivity = 93%, specificity = 100%) in differentiating neoplastic disease from treatment-related changes by combining static and dynamic information from FET scans [129]. In a similar-sized study of 110 patients, performances of FDOPA PET were slightly lower, with an accuracy of 82% (sensitivity = 89.6%, specificity = 72.4%) [130]. The results of these two latter studies, however, are hardly comparable because of the different methods of image analysis and of an unbalanced percentage of recurrent diseases and unspecific changes in the two patient cohorts. Another study using F-DOPA PET in a smaller population of 35 patients showed an overall accuracy of 97% in the same setting [131]. Using MET PET, Terakawa and coworkers studied a heterogeneous sample of 26 gliomas showing both sensitivity and specificity of 75% for discriminating between tumour recurrence and radiation necrosis [132]. In a later study, D’Souza and coworkers found an overall accuracy of 89.6% (sensitivity = 94.7%, specificity = 80%) in 29 patients with high-grade gliomas [133]. The same group has recently extended these results to a larger cohort of 64 tumours from various histologies, particularly emphasizing the higher accuracy of MET over FDG PET in low-grade gliomas (sensitivity = 93.3% specificity = 90%) [134].
The performances of PWI techniques (either DSC or DCE) in correct classification of late treatment-related changes were assessed by several studies, resulting in cumulated sensitivity and specificity of 90 and 88%, respectively [126, 135–138]. Of note, the presence of haemorrhage, image distortion, and susceptibility artifacts limits the applicability of DSC perfusion MR images in a non-negligible number of cases [136, 139]. In some of these non-interpretable cases, however, DCE MRI seems to overcome the limitations of DSC images [139]. Some studies have compared amino acid PET vs PWI and reported on a similar diagnostic power of PWI in this setting, but the number of patients in those studies is relatively small, so that currently, no reliable conclusion can be drawn [133, 140, 141] (Table 1).
Amino acid PET imaging has also proved to be valuable in discriminating between tumour recurrence and radionecrosis in brain metastases. Radionecrosis occurs after a median of 12 months after radiation treatment and its incidence varies depending on the local radiation dose [142, 143]. Using FET PET, the combination of static imaging with dynamic information yielded an overall accuracy ranging between 87 and 93% in two different patient cohorts with a total of 76 [28, 144] and 34 brain metastases, respectively [145]. Encouraging results were also obtained with FDOPA in 83 brain metastases using a qualitative approach for image analysis (sensitivity = 81.3%, specificity = 84.3%) [146] and in another study in 50 brain metastases using a semiquantitative approach for image analysis (sensitivity = 90%, specificity = 92.3%, accuracy = 91.3%) [147]. In addition, this latter paper reports on the only available direct comparison between amino acid PET and PWI in this setting, demonstrating better performances of amino acid PET in classifying indeterminate enlarging brain metastases after radiation treatment (37 lesions available for comparison, 91.9 vs 75.6% overall accuracy for FDOPA and DSC PWI, respectively). Recent data from the same group confirmed the superior performances of F-DOPA PET over MRI including DSC PWI techniques in assessing the evolution over time of predominantly radionecrotic brain metastases after stereotactic radiosurgery [148]. As regards MET, available data suggest moderately lower discriminating power (sensitivity = 79%, specificity = 75%) in 51 secondary lesions [132]. PWI holds promises in the setting of brain metastases as well, albeit the number of available studies and patients included is limited [149–151]. At present, a combined approach for differential diagnosis is to be encouraged, as already put in practice by single specialized centers [152, 153].
In summary, available literature shows that both amino acid PET and perfusion-weighted MRI are useful aids to the differential diagnosis between tumour progression and treatment-related changes in both gliomas and brain metastases; hence, an integrated approach is advised.
Amino acid PET and MR perfusion imaging for treatment monitoring of brain tumours
The early detection of tumour response to therapy is of great importance for the optimization of individual tumor therapy.
In the context of both chemoradiation with temozolomide and temozolomide monotherapy, the reliability of treatment monitoring using MET was shown in a cohort of 15 patients with heterogeneous tumour histologies and prior treatment history [154]. Using FET PET, Piroth et al. demonstrated that an early (7–10 days after treatment completion) >10% reduction of TBRmax from baseline was predictor of disease free survival and overall survival in a homogeneous population of 22 patients with glioblastoma, whereas no such prognostic significance could be found for changes in contrast enhancement [107, 155]. In addition, FET PET demonstrated the ability to stratify responses to temozolomide several months earlier than MRI in 11 patients with progressive non-enhancing low-grade gliomas [156]. More recently, a multicenter study demonstrated that changes of tumour volume in amino acid PET were superior to MRI for evaluating responses to temozolomide in WHO grade II glioma and to predict progression-free survival [157].
Amino acid PET tracers were also tested in the response evaluation of antiangiogenic treatments. A special problem represents the so-called “pseudoresponse” during antiangiogenic treatment. Here, a fast reduction of contrast enhancement in MRI may hide the underlying growth of the non-enhancing, T2-positive, portion of the tumour. The sensitivity and reliability of the conventional MRI in this setting is limited [4]. In a cohort of 11 patients under bevacizumab treatment, a significant discrepancy between FET uptake and RANO criteria was observed in 4/11 patients suggesting that FET can detect treatment failure earlier than the conventional MRI [158]. Similar findings were subsequently reported in an independent cohort of ten patients treated with bevacizumab [159]. Using FDOPA PET, metabolically active tumour volume measured as early as 2 weeks after therapy initiation, as well as tumour volume changes during therapy, was also found to be strong predictors of survival in a larger cohort of 30 patients under bevacizumab/irinotecan [160]. In that study, there were eight (26%) discordant cases and PET outperformed MRI-based RANO criteria.
A few studies have evaluated the performances of PWI in early treatment assessment. Unfortunately, up to now, no direct comparison with amino acid PET exists. Sawlani and colleagues have retrospectively evaluated 16 patients with glioblastoma undergoing bevacizumab treatment, deriving an index of local perfusion (hyperperfusion volume, HPV) which correlated with time-to-progression [161]. The largest study available included 36 patients with high-grade gliomas who underwent perfusion-weighted sequences in addition to standard MRI before and during bevacizumab treatment [162]. In that study, an improved survival was obtained in patients with low tumour rCBV either before or during treatment. In contrast, a study evaluating the effect of cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, showed that an increase in tumour perfusion was predictive for better outcome [163], according to the “vascular normalization hypothesis” [164].
In summary, amino acid PET shows convincing results concerning sensitive and specific assessment of treatment response at an early stage during chemotherapy or antiangiogenic therapy. In contrast, despite widespread availability, PWI has so far only been investigated to a small extent in this area and further investigations are needed.
Conclusion
In contrast to amino acid PET, most neuro oncological centres have access to PWI which, however, requires a great deal of experience to ensure meaningful assessment are made and therefore is not always used efficiently. Another limitation of PWI is the lack of standardization of the methods used and in data processing. Amino acid PET is as yet not available in every centre, but the method is rapidly spreading not only because of its diagnostic power but also because of its robustness and the fact that interpreting amino acid PET may be easier for clinicians involved in neuro oncology than PWI owing to the higher tumour to background contrast and more homogenous background. The two methods are based on different biochemical-physiological mechanisms but can provide diagnostic information beyond the conventional MRI depending on the clinical question.
At initial diagnosis of space occupying brain lesions, a relatively reliable differential diagnosis can be achieved by the use of the conventional MRI and PWI, and amino acid PET appears necessary in equivocal situations only. In the case of highly suspicious lesions, both methods can help to define a site for biopsy to obtain a meaningful histology. Amino acid PET, on the other hand, appears to be more powerful to define the tumor extent (Table 2). If, after the primary therapy of the tumour, there is a suspicion of progression or recurrence, both methods are helpful to differentiate these from unspecific posttherapeutic changes. An early and sensitive assessment of therapeutic efficacy can be achieved especially with amino acid PET, while the data with PWI are sparse despite a broad availability. To further improve the diagnostics of brain tumours, it seems necessary to explore the complementary nature of these two methods in further studies. This is not only in the interest of the patients, but also of society, since the treatment costs for this disease are extremely high.
References
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J et al (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(Suppl 4):iv1–iv63
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14:48–54
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM et al (2016) Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 18:1199–1208
Galldiks N, Langen KJ, Pope WB (2015) From the clinician’s point of view—What is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol 17:1434–1444
Langstrom B, Antoni G, Gullberg P, Halldin C, Malmborg P, Nagren K et al (1987) Synthesis of l- and d-[methyl-11C]methionine. J Nucl Med 28:1037–1040
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M et al (1999) Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-l-tyrosine for tumor imaging. J Nucl Med 40:205–212
Luxen A, Guillaume M, Melega WP, Pike VW, Solin O, Wagner R (1992) Production of 6-[18F]fluoro-l-dopa and its metabolism in vivo—a critical review. Int J Rad Appl Instrum B 19:149–158
Lemaire C, Libert L, Franci X, Genon JL, Kuci S, Giacomelli F et al (2015) Automated production at the curie level of no-carrier-added 6-[(18)F]fluoro-l-dopa and 2-[(18)F]fluoro-l-tyrosine on a FASTlab synthesizer. J Label Comp Radiopharm 58:281–290
Juhasz C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S (2014) Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging 13:1–16
Nowosielski M, DiFranco MD, Putzer D, Seiz M, Recheis W, Jacobs AH et al (2014) An intra-individual comparison of MRI, [18F]-FET and [18F]-FLT PET in patients with high-grade gliomas. PLoS ONE 9:e95830
Kato T, Shinoda J, Nakayama N, Miwa K, Okumura A, Yano H et al (2008) Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. Am J Neuroradiol 29:1176–1182
Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T (2010) Correlation of l-methyl-11C-methionine (MET) uptake with l-type amino acid transporter 1 in human gliomas. J Neurooncol 99:217–225
Youland RS, Kitange GJ, Peterson TE, Pafundi DH, Ramiscal JA, Pokorny JL et al (2013) The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol 111:11–18
Langen KJ, Hamacher K, Weckesser M, Floeth F, Stoffels G, Bauer D et al (2006) O-(2-[18F]fluoroethyl)-l-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol 33:287–294
Habermeier A, Graf J, Sandhofer BF, Boissel JP, Roesch F, Closs EI (2015) System l amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-l-tyrosine (FET). Amino Acids 47:335–344
Becherer A, Karanikas G, Szabo M, Zettinig G, Asenbaum S, Marosi C et al (2003) Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging 30:1561–1567
Grosu AL, Astner ST, Riedel E, Nieder C, Wiedenmann N, Heinemann F et al (2011) An interindividual comparison of O-(2-[(18)F]fluoroethyl)-l-tyrosine (FET)- and l-[Methyl-(11)C]Methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys 81:1049–1058
Kratochwil C, Combs SE, Leotta K, Afshar-Oromieh A, Rieken S, Debus J et al (2014) Intra-individual comparison of (18)F-FET and (18)F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol 16:434–440
Lapa C, Linsenmann T, Monoranu CM, Samnick S, Buck AK, Bluemel C et al (2014) Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med 55:1611–1616
Schiepers C, Chen W, Cloughesy T, Dahlbom M, Huang SC (2007) 18F-FDOPA kinetics in brain tumors. J Nucl Med 48:1651–1661
Cicone F, Filss CP, Minniti G, Rossi-Espagnet C, Papa A, Scaringi C et al (2015) Volumetric assessment of recurrent or progressive gliomas: comparison between F-DOPA PET and perfusion-weighted MRI. Eur J Nucl Med Mol Imaging 42:905–915
Calcagni ML, Galli G, Giordano A, Taralli S, Anile C, Niesen A et al (2011) Dynamic O-(2-[18F]fluoroethyl)-l-tyrosine (F-18 FET) PET for glioma grading: assessment of individual probability of malignancy. Clin Nucl Med 36:841–847
Albert NL, Winkelmann I, Suchorska B, Wenter V, Schmid-Tannwald C, Mille E et al (2016) Early static (18)F-FET-PET scans have a higher accuracy for glioma grading than the standard 20–40 min scans. Eur J Nucl Med Mol Imaging 43:1105–1114
Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M et al (2015) Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[(18)F]fluoroethyl)-l-tyrosine PET. Eur J Nucl Med Mol Imaging 42:685–695
Galldiks N, Stoffels G, Filss C, Rapp M, Blau T, Tscherpel C et al (2015) The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol 17:1293–1300
Ceccon G, Lohmann P, Stoffels G, Judov N, Filss CP, Rapp M et al (2016) Dynamic O-(2-18F-fluoroethyl)-l-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. doi:10.1093/neuonc/now149
Jansen NL, Suchorska B, Wenter V, Schmid-Tannwald C, Todica A, Eigenbrod S et al (2015) Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J Nucl Med 56:9–15
Jansen NL, Suchorska B, Wenter V, Eigenbrod S, Schmid-Tannwald C, Zwergal A et al (2014) Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J Nucl Med 55:198–203
Dunet V, Pomoni A, Hottinger A, Nicod-Lalonde M, Prior JO (2016) Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol 18:426–434
Floeth FW, Pauleit D, Sabel M, Reifenberger G, Stoffels G, Stummer W et al (2006) 18F-FET PET differentiation of ring-enhancing brain lesions. J Nucl Med 47:776–782
Herholz K, Holzer T, Bauer B, Schroder R, Voges J, Ernestus RI et al (1998) 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology 50:1316–1322
Hutterer M, Nowosielski M, Putzer D, Jansen NL, Seiz M, Schocke M et al (2013) [F-18]-fluoro-ethyl-l-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol 15:341–351
Rapp M, Heinzel A, Galldiks N, Stoffels G, Felsberg J, Ewelt C et al (2013) Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med 54:229–235
Sala Q, Metellus P, Taieb D, Kaphan E, Figarella-Branger D, Guedj E (2014) 18F-DOPA, a clinically available PET tracer to study brain inflammation? Clin Nucl Med 39:e283–e285
Unterrainer M, Schweisthal F, Suchorska B, Wenter V, Schmid-Tannwald C, Fendler WP et al (2016) Serial 18F-FET PET imaging of primarily 18F-FET-negative glioma—does it make sense? J Nucl Med 57(8):1177–1182. doi:10.2967/jnumed.115.171033
Floeth FW, Pauleit D, Sabel M, Stoffels G, Reifenberger G, Riemenschneider MJ et al (2007) Prognostic value of O-(2-18F-fluoroethyl)-l-tyrosine PET and MRI in low-grade glioma. J Nucl Med 48:519–527
Cha S (2009) Neuroimaging in neuro-oncology. Neurotherapeutics 6:465–477
Hutterer M, Hattingen E, Palm C, Proescholdt MA, Hau P (2015) Current standards and new concepts in MRI and PET response assessment of antiangiogenic therapies in high-grade glioma patients. Neuro Oncol 17:784–800
Ellingson BM, Bendszus M, Boxerman J, Barboriak D, Erickson BJ, Smits M et al (2015) Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol 17:1188–1198
Prabhakar R, Haresh KP, Ganesh T, Joshi RC, Julka PK, Rath GK (2007) Comparison of computed tomography and magnetic resonance based target volume in brain tumors. J Cancer Res Ther 3:121–123
Jansen EP, Dewit LG, van Herk M, Bartelink H (2000) Target volumes in radiotherapy for high-grade malignant glioma of the brain. Radiother Oncol 56:151–156
Herholz K, Coope D, Jackson A (2007) Metabolic and molecular imaging in neuro-oncology. Lancet Neurol 6:711–724
Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD et al (2005) Comparative overview of brain perfusion imaging techniques. Stroke 36:e83–e99
Covarrubias DJ, Rosen BR, Lev MH (2004) Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist 9:528–537
Jackson A, O’Connor J, Thompson G, Mills S (2008) Magnetic resonance perfusion imaging in neuro-oncology. Cancer Imaging 8:186–199
Paulson ES, Schmainda KM (2008) Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology 249:601–613
Knutsson L, Lindgren E, Ahlgren A, van Osch MJ, Bloch KM, Surova Y et al (2014) Dynamic susceptibility contrast MRI with a prebolus contrast agent administration design for improved absolute quantification of perfusion. Magn Reson Med 72:996–1006
Quarles CC, Gore JC, Xu L, Yankeelov TE (2012) Comparison of dual-echo DSC-MRI- and DCE-MRI-derived contrast agent kinetic parameters. Magn Reson Imaging 30:944–953
Skinner JT, Robison RK, Elder CP, Newton AT, Damon BM, Quarles CC (2014) Evaluation of a multiple spin- and gradient-echo (SAGE) EPI acquisition with SENSE acceleration: applications for perfusion imaging in and outside the brain. Magn Reson Imaging 32:1171–1180
Lemasson B, Valable S, Farion R, Krainik A, Remy C, Barbier EL (2013) In vivo imaging of vessel diameter, size, and density: a comparative study between MRI and histology. Magn Reson Med 69:18–26
Emblem KE, Mouridsen K, Bjornerud A, Farrar CT, Jennings D, Borra RJ et al (2013) Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med 19:1178–1183
Svolos P, Kousi E, Kapsalaki E, Theodorou K, Fezoulidis I, Kappas C et al (2014) The role of diffusion and perfusion weighted imaging in the differential diagnosis of cerebral tumors: a review and future perspectives. Cancer Imaging 14:20
Kebir S, Gaertner FC, Mueller M, Nelles M, Simon M, Schafer N et al (2016) 18F-fluoroethyl-l-tyrosine positron emission tomography for the differential diagnosis of tumefactive multiple sclerosis versus glioma: a case report. Oncol Lett 11:2195–2198
Dunet V, Rossier C, Buck A, Stupp R, Prior JO (2012) Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nucl Med 53:207–214
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M et al (2004) Delineation of brain tumor extent with [11C]l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 10:7163–7170
Lopez WO, Cordeiro JG, Albicker U, Doostkam S, Nikkhah G, Kirch RD et al (2015) Correlation of (18)F-fluoroethyl tyrosine positron-emission tomography uptake values and histomorphological findings by stereotactic serial biopsy in newly diagnosed brain tumors using a refined software tool. Onco Targets Ther 8:3803–3815
Mosskin M, Ericson K, Hindmarsh T, von Holst H, Collins VP, Bergstrom M et al (1989) Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol 30:225–232
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW et al (2005) O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128:678–687
Pafundi DH, Laack NN, Youland RS, Parney IF, Lowe VJ, Giannini C et al (2013) Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol 15:1058–1067
Arbizu J, Tejada S, Marti-Climent JM, Diez-Valle R, Prieto E, Quincoces G et al (2012) Quantitative volumetric analysis of gliomas with sequential MRI and (11)C-methionine PET assessment: patterns of integration in therapy planning. Eur J Nucl Med Mol Imaging 39:771–781
Grosu AL, Weber WA (2010) PET for radiation treatment planning of brain tumours. Radiother Oncol 96:325–327
Piroth MD, Pinkawa M, Holy R, Stoffels G, Demirel C, Attieh C et al (2009) Integrated-boost IMRT or 3-D-CRT using FET-PET based auto-contoured target volume delineation for glioblastoma multiforme—a dosimetric comparison. Radiat Oncol 4:57
Pirotte BJ, Levivier M, Goldman S, Massager N, Wikler D, Dewitte O et al (2009) Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 64:471–481 (discussion 81)
Pirotte BJ, Lubansu A, Massager N, Wikler D, Van Bogaert P, Levivier M et al (2010) Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr 5:486–499
Matsuo M, Miwa K, Tanaka O, Shinoda J, Nishibori H, Tsuge Y et al (2012) Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys 82:83–89
Miwa K, Matsuo M, Shinoda J, Oka N, Kato T, Okumura A et al (2008) Simultaneous integrated boost technique by helical tomotherapy for the treatment of glioblastoma multiforme with 11C-methionine PET: report of three cases. J Neurooncol 87:333–339
Rickhey M, Moravek Z, Eilles C, Koelbl O, Bogner L (2010) 18F-FET-PET-based dose painting by numbers with protons. Strahlenther Onkol 186:320–326
Rieken S, Habermehl D, Giesel FL, Hoffmann C, Burger U, Rief H et al (2013) Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol 109:487–492
Munck P, Rosenschold AF, Costa J, Engelholm SA, Lundemann MJ, Law I, Ohlhues L et al (2015) Impact of [18F]-fluoro-ethyl-tyrosine PET imaging on target definition for radiation therapy of high-grade glioma. Neuro Oncol 17:757–763
Niyazi M, Jansen N, Ganswindt U, Schwarz SB, Geisler J, Schnell O et al (2012) Re-irradiation in recurrent malignant glioma: prognostic value of [18F]FET-PET. J Neurooncol 110:389–395
Navarria P, Reggiori G, Pessina F, Ascolese AM, Tomatis S, Mancosu P et al (2014) Investigation on the role of integrated PET/MRI for target volume definition and radiotherapy planning in patients with high grade glioma. Radiother Oncol 112:425–429
Price SJ, Green HA, Dean AF, Joseph J, Hutchinson PJ, Gillard JH (2011) Correlation of MR relative cerebral blood volume measurements with cellular density and proliferation in high-grade gliomas: an image-guided biopsy study. Am J Neuroradiol 32:501–506
Blasel S, Franz K, Ackermann H, Weidauer S, Zanella F, Hattingen E (2011) Stripe-like increase of rCBV beyond the visible border of glioblastomas: site of tumor infiltration growing after neurosurgery. J Neurooncol 103:575–584
Sadeghi N, D’Haene N, Decaestecker C, Levivier M, Metens T, Maris C et al (2008) Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. Am J Neuroradiol 29:476–482
Sadeghi N, Salmon I, Decaestecker C, Levivier M, Metens T, Wikler D et al (2007) Stereotactic comparison among cerebral blood volume, methionine uptake, and histopathology in brain glioma. Am J Neuroradiol 28:455–461
Sadeghi N, Salmon I, Tang BN, Denolin V, Levivier M, Wikler D et al (2006) Correlation between dynamic susceptibility contrast perfusion MRI and methionine metabolism in brain gliomas: preliminary results. J Magn Reson Imaging 24:989–994
Filss CP, Galldiks N, Stoffels G, Sabel M, Wittsack HJ, Turowski B et al (2014) Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J Nucl Med 55:540–545
Henriksen OM, Larsen VA, Muhic A, Hansen AE, Larsson HB, Poulsen HS et al (2016) Simultaneous evaluation of brain tumour metabolism, structure and blood volume using [(18)F]-fluoroethyltyrosine (FET) PET/MRI: feasibility, agreement and initial experience. Eur J Nucl Med Mol Imaging 43:103–112
Gottler J, Lukas M, Kluge A, Kaczmarz S, Gempt J, Ringel F et al (2017) Intra-lesional spatial correlation of static and dynamic FET-PET parameters with MRI-based cerebral blood volume in patients with untreated glioma. Eur J Nucl Med Mol Imaging 44:392–397
Tietze A, Boldsen JK, Mouridsen K, Ribe L, Dyve S, Cortnum S et al (2015) Spatial distribution of malignant tissue in gliomas: correlations of C-11-l-methionine positron emission tomography and perfusion- and diffusion-weighted magnetic resonance imaging. Acta Radiol 56:1135–1144
Berntsson SG, Falk A, Savitcheva I, Godau A, Zetterling M, Hesselager G et al (2013) Perfusion and diffusion MRI combined with (1)(1)C-methionine PET in the preoperative evaluation of suspected adult low-grade gliomas. J Neurooncol 114:241–249
Pauleit D, Stoffels G, Bachofner A, Floeth FW, Sabel M, Herzog H et al (2009) Comparison of (18)F-FET and (18)F-FDG PET in brain tumors. Nucl Med Biol 36:779–787
Pirotte B, Goldman S, Massager N, David P, Wikler D, Lipszyc M et al (2004) Combined use of 18F-fluorodeoxyglucose and 11C-methionine in 45 positron emission tomography-guided stereotactic brain biopsies. J Neurosurg 101:476–483
Plotkin M, Blechschmidt C, Auf G, Nyuyki F, Geworski L, Denecke T et al (2010) Comparison of F-18 FET-PET with F-18 FDG-PET for biopsy planning of non-contrast-enhancing gliomas. Eur Radiol 20:2496–2502
Galldiks N, Stoffels G, Ruge MI, Rapp M, Sabel M, Reifenberger G et al (2013) Role of O-(2-18F-fluoroethyl)-l-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med 54:2046–2054
Kunz M, Thon N, Eigenbrod S, Hartmann C, Egensperger R, Herms J et al (2011) Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol 13:307–316
Thon N, Kunz M, Lemke L, Jansen NL, Eigenbrod S, Kreth S et al (2015) Dynamic F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int J Cancer 136:2132–2145
Dunet V, Prior JO (2016) Response to: performance of 18F-FET-PET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: inherent bias in meta-analysis not revealed by quality metrics. Neuro Oncol 18:1029–1030
Kracht LW, Friese M, Herholz K, Schroeder R, Bauer B, Jacobs A et al (2003) Methyl-[11C]- l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging 30:868–873
Manabe O, Hattori N, Yamaguchi S, Hirata K, Kobayashi K, Terasaka S et al (2015) Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging. Eur J Nucl Med Mol Imaging 42:896–904
Pöpperl G, Kreth FW, Mehrkens JH, Herms J, Seelos K, Koch W et al (2007) FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging 34:1933–1942
Jansen NL, Schwartz C, Graute V, Eigenbrod S, Lutz J, Egensperger R et al (2012) Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [(18)F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro Oncol 14:1473–1480
Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J (2008) 11C-l-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol 10:1–18
Pöpperl G, Kreth FW, Herms J, Koch W, Mehrkens JH, Gildehaus FJ et al (2006) Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J Nucl Med 47:393–403
Weckesser M, Langen KJ, Rickert CH, Kloska S, Straeter R, Hamacher K et al (2005) O-(2-[18F]fluorethyl)-l-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging 32:422–429
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. Am J Neuroradiol 24:1989–1998
Arvinda HR, Kesavadas C, Sarma PS, Thomas B, Radhakrishnan VV, Gupta AK et al (2009) Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging. J Neurooncol 94:87–96
Toyooka M, Kimura H, Uematsu H, Kawamura Y, Takeuchi H, Itoh H (2008) Tissue characterization of glioma by proton magnetic resonance spectroscopy and perfusion-weighted magnetic resonance imaging: glioma grading and histological correlation. Clin Imaging 32:251–258
Hilario A, Ramos A, Perez-Nunez A, Salvador E, Millan JM, Lagares A et al (2012) The added value of apparent diffusion coefficient to cerebral blood volume in the preoperative grading of diffuse gliomas. Am J Neuroradiol 33:701–707
Fayed N, Davila J, Medrano J, Olmos S (2008) Malignancy assessment of brain tumours with magnetic resonance spectroscopy and dynamic susceptibility contrast MRI. Eur J Radiol 67:427–433
Lev M, Ozsunar Y, Henson J et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. Am J Neuroradiol 25:214–221
Guzman-De-Villoria JA, Mateos-Perez JM, Fernandez-Garcia P, Castro E, Desco M (2014) Added value of advanced over conventional magnetic resonance imaging in grading gliomas and other primary brain tumors. Cancer Imaging 14:35
Usinskiene J, Ulyte A, Bjornerud A, Venius J, Katsaros VK, Rynkeviciene R et al (2016) Optimal differentiation of high- and low-grade glioma and metastasis: a meta-analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology 58:339–350
Galldiks N, Dunkl V, Kracht LW, Vollmar S, Jacobs AH, Fink GR et al (2012) Volumetry of [(1)(1)C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol Imaging 11:516–527
Piroth MD, Pinkawa M, Holy R, Klotz J, Nussen S, Stoffels G et al (2011) Prognostic value of early [18F]fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int J Radiat Oncol Biol Phys 80:176–184
Suchorska B, Jansen NL, Linn J, Kretzschmar H, Janssen H, Eigenbrod S et al (2015) Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 84:710–719
Ribom D, Eriksson A, Hartman M, Engler H, Nilsson A, Langstrom B et al (2001) Positron emission tomography (11)C-methionine and survival in patients with low-grade gliomas. Cancer 92:1541–1549
Smits A, Westerberg E, Ribom D (2008) Adding 11C-methionine PET to the EORTC prognostic factors in grade 2 gliomas. Eur J Nucl Med Mol Imaging 35:65–71
Smits A, Baumert BG (2011) The clinical value of PET with amino acid tracers for gliomas WHO Grade II. Int J Mol Imaging 2011:372509
Rapp M, Floeth FW, Felsberg J, Steiger HJ, Sabel M, Langen KJ et al (2013) Clinical value of O-(2-[(18)F]-fluoroethyl)-l-tyrosine positron emission tomography in patients with low-grade glioma. Neurosurg Focus 34:E3
Villani V, Carapella CM, Chiaravalloti A, Terrenato I, Piludu F, Vidiri A et al (2015) The role of PET [18F]FDOPA in evaluating low-grade glioma. Anticancer Res 35:5117–5122
Jansen NL, Graute V, Armbruster L, Suchorska B, Lutz J, Eigenbrod S et al (2012) MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging 39:1021–1029
Hirai T, Murakami R, Nakamura H, Kitajima M, Fukuoka H, Sasao A et al (2008) Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. Am J Neuroradiol 29:1505–1510
Jain R, Poisson L, Narang J, Gutman D, Scarpace L, Hwang SN et al (2013) Genomic mapping and survival prediction in glioblastoma: molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology 267:212–220
Shiroishi MS, Boxerman JL, Pope WB (2016) Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Neuro Oncol 18:467–478
Rossi Espagnet MC, Romano A, Mancuso V, Cicone F, Napolitano A, Scaringi C et al (2016) Multiparametric evaluation of low grade gliomas at follow-up: comparison between diffusion and perfusion MR with (18)F-FDOPA PET. Br J Radiol 89:20160476
Walker AJ, Ruzevick J, Malayeri AA, Rigamonti D, Lim M, Redmond KJ et al (2014) Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol 10:1277–1297
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197
Kong DS, Kim ST, Kim EH, Lim DH, Kim WS, Suh YL et al (2011) Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: the role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. Am J Neuroradiol 32:382–387
Baek HJ, Kim HS, Kim N, Choi YJ, Kim YJ (2012) Percent change of perfusion skewness and kurtosis: a potential imaging biomarker for early treatment response in patients with newly diagnosed glioblastomas. Radiology 264:834–843
Choi YJ, Kim HS, Jahng GH, Kim SJ, Suh DC (2013) Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol 54:448–454
Cha J, Kim ST, Kim HJ, Kim BJ, Kim YK, Lee JY et al (2014) Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. Am J Neuroradiol 35:1309–1317
Prager AJ, Martinez N, Beal K, Omuro A, Zhang Z, Young RJ (2015) Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. Am J Neuroradiol 36:877–885
Patel P, Baradaran H, Delgado D, Askin G, Christos P, Tsiouris AJ et al (2017) MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol 9:118–127
Suh CH, Kim HS, Choi YJ, Kim N, Kim SJ (2013) Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. Am J Neuroradiol 34:2278–2286
Rachinger W, Goetz C, Popperl G, Gildehaus FJ, Kreth FW, Holtmannspotter M et al (2005) Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57:505–511 (discussion-11)
Galldiks N, Stoffels G, Filss C, Rapp M, Blau T, Tscherpel C et al (2015) The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol 17:1293–1300
Herrmann K, Czernin J, Cloughesy T, Lai A, Pomykala KL, Benz MR et al (2014) Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol 16:603–609
Karunanithi S, Sharma P, Kumar A, Khangembam BC, Bandopadhyaya GP, Kumar R et al (2013) Comparative diagnostic accuracy of contrast-enhanced MRI and (18)F-FDOPA PET-CT in recurrent glioma. Eur Radiol 23:2628–2635
Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T et al (2008) Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49:694–699
D’Souza MM, Sharma R, Jaimini A, Panwar P, Saw S, Kaur P et al (2014) 11C-MET PET/CT and advanced MRI in the evaluation of tumor recurrence in high-grade gliomas. Clin Nucl Med 39:791–798
Sharma R, D’Souza M, Jaimini A, Hazari PP, Saw S, Pandey S et al (2016) A comparison study of (11)C-methionine and (18)F-fluorodeoxyglucose positron emission tomography-computed tomography scans in evaluation of patients with recurrent brain tumors. Indian J Nucl Med 31:93–102
Barajas RF Jr, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS et al (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM et al (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. Am J Neuroradiol 30:552–558
Gasparetto EL, Pawlak MA, Patel SH, Huse J, Woo JH, Krejza J et al (2009) Posttreatment recurrence of malignant brain neoplasm: accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology 250:887–896
Chung WJ, Kim HS, Kim N, Choi CG, Kim SJ (2013) Recurrent glioblastoma: optimum area under the curve method derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. Radiology 269:561–568
Heo YJ, Kim HS, Park JE, Choi CG, Kim SJ (2015) Uninterpretable dynamic susceptibility contrast-enhanced perfusion MR images in patients with post-treatment glioblastomas: cross-validation of alternative imaging options. PLoS ONE 10:e0136380
Dandois V, Rommel D, Renard L, Jamart J, Cosnard G (2010) Substitution of 11C-methionine PET by perfusion MRI during the follow-up of treated high-grade gliomas: preliminary results in clinical practice. J Neuroradiol 37:89–97
Kim YH, Oh SW, Lim YJ, Park CK, Lee SH, Kang KW et al (2010) Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg 112:758–765
Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC (2010) Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 77:996–1001
Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48
Galldiks N, Stoffels G, Filss CP, Piroth MD, Sabel M, Ruge MI et al (2012) Role of O-(2-18F-fluoroethyl)-l-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 53:1367–1374
Romagna A, Unterrainer M, Schmid-Tannwald C, Brendel M, Tonn JC, Nachbichler SB et al (2016) Suspected recurrence of brain metastases after focused high dose radiotherapy: can [18F]FET-PET overcome diagnostic uncertainties? Radiat Oncol 11:139
Lizarraga KJ, Allen-Auerbach M, Czernin J, DeSalles AA, Yong WH, Phelps ME et al (2014) (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med 55:30–36
Cicone F, Minniti G, Romano A, Papa A, Scaringi C, Tavanti F et al (2015) Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging 42:103–111
Cicone FCL, Scaringi C, Romano A, Bozzao A, Minniti G, Scopinaro F (2016) Value of F-DOPA PET in the long term follow up of radionecrotic brain metastases after radiosurgery: comparison with MRI. Eur J Nucl Med Mol Imaging 43(Suppl1):195–196
Hoefnagels FW, Lagerwaard FJ, Sanchez E, Haasbeek CJ, Knol DL, Slotman BJ et al (2009) Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 256:878–887
Mitsuya K, Nakasu Y, Horiguchi S, Harada H, Nishimura T, Bando E et al (2010) Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol 99:81–88
Hatzoglou V, Yang TJ, Omuro A, Gavrilovic I, Ulaner G, Rubel J et al (2016) A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol 18:873–880
Minniti G, Scaringi C, Paolini S, Clarke E, Cicone F, Esposito V et al (2016) Repeated stereotactic radiosurgery for patients with progressive brain metastases. J Neurooncol 126:91–97
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F et al (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148
Galldiks N, Kracht LW, Burghaus L, Thomas A, Jacobs AH, Heiss WD et al (2006) Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur J Nucl Med Mol Imaging 33:516–524
Galldiks N, Langen K, Holy R, Pinkawa M, Stoffels G, Nolte K et al (2012) Assessment of treatment response in patients with glioblastoma using [18F]Fluoroethyl-l-Tyrosine PET in comparison to MRI. J Nucl Med 53:1048–1057
Wyss M, Hofer S, Bruehlmeier M, Hefti M, Uhlmann C, Bartschi E et al (2009) Early metabolic responses in temozolomide treated low-grade glioma patients. J Neurooncol 95:87–93
Roelcke U, Wyss MT, Nowosielski M, Ruda R, Roth P, Hofer S et al (2016) Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro Oncol 18:744–751
Hutterer M, Nowosielski M, Putzer D, Waitz D, Tinkhauser G, Kostron H et al (2011) O-(2-18F-fluoroethyl)-l-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med 52:856–864
Galldiks N, Rapp M, Stoffels G, Fink GR, Shah NJ, Coenen HH et al (2013) Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-l-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging 40:22–33
Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Grogan T et al (2014) Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res 20:3550–3559
Sawlani RN, Raizer J, Horowitz SW, Shin W, Grimm SA, Chandler JP et al (2010) Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging—pilot study. Radiology 255:622–628
Schmainda KM, Prah M, Connelly J, Rand SD, Hoffman RG, Mueller W et al (2014) Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol 16:880–888
Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M et al (2012) Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res 72:402–407
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7:987–989
Author information
Authors and Affiliations
Contributions
CF: Literature Search, Manuscript writing and Editing, critical revision for important intellectual content, final approval of the version to be published. FC: Literature Search, Manuscript writing and Editing, critical revision for important intellectual content, final approval of the version to be published. NJS: Literature Search, Manuscript writing and Editing, critical revision for important intellectual content, final approval of the version to be published. NG: Literature Search, Manuscript writing and Editing, critical revision for important intellectual content, final approval of the version to be published. KJL: Content planning and Literature Search, Manuscript writing and Editing, critical revision for important intellectual content, final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Role of the funding source
Prof. Shah has received funding from the EC Seventh Framework Programme (European Union)—Project No. 602621.
Ethical approval
The article is a literature review for which no ethical approval is required.
Informed consent
The article is a literature review for which no additional patients were investigated.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s40336-017-0239-6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Filss, C.P., Cicone, F., Shah, N.J. et al. Amino acid PET and MR perfusion imaging in brain tumours. Clin Transl Imaging 5, 209–223 (2017). https://doi.org/10.1007/s40336-017-0225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-017-0225-z