Abstract
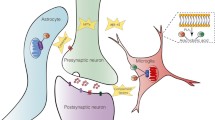
Neuroinflammatory changes are observed in the brain of patients with Alzheimer’s disease (AD). Studies have shown the presence of activated microglia and astrocytes surrounding the amyloid plaques, along with the presence of cytokines and other mediators of inflammation. The role of inflammation in AD is not yet completely understood. More specifically, some inflammatory processes, such as the activation of microglia, may have detrimental or beneficial effects on the underlining neuropathology, by promoting inflammation and tissue damage or rather phagocytic activity and tissue repair. Imaging of neuroinflammation with positron emission tomography (PET) is the only technology that enables the visualization of microglia and astrocyte activation in the living human brain. PET studies with first- or second-generation radioligands binding to the 18-kDa translocator protein (TSPO) ([11C]-R-PK11195, [11C]DAA1106, [11C]PBR28, [18F]FEMPA, [18F]FEPPA) have shown some conflicting results, demonstrating on average a ~30 % higher TSPO availability in AD patients compared with controls, with a few studies showing no statistically significant difference between the two groups. Similar conflicting evidences have been shown when comparing subjects with mild cognitive impairment (MCI) and control subjects. Therefore, whether TSPO is a good marker for detecting in vivo microglia activation in AD is still a matter of debate. Imaging of MAO-B as a marker for astrocyte activation in AD is a valid alternative to TSPO imaging in the context of neuroinflammation. Only limited MAO-B imaging studies with [11C]l-deprenyl-D2 are available so far in AD and MCI, showing increased MAO-B binding in MCI patients compared with controls with a degree higher than that observed in AD. There are two unmet questions that are still under discussion. The first question is which neuroinflammatory process, microglia or astrocyte activation, occurs earlier in the natural course of AD from prodromal to dementia stage? Comparative studies using these two markers in MCI and AD could be important to clarify which marker can be used for earliest detection of neuroinflammatory changes in vivo. The second question is whether imaging of microglia or astrocytes per se is a useful marker of neuroinflammation associated with neurodegeneration. The development of new radioligands for other targets that are more directly associated with the pro- or anti-inflammatory activity of microglia could help in understanding the relevance of neuroinflammation in the pathological processes leading to neurodegeneration in AD. Molecular imaging with PET can be a useful tool to determine the nature and temporal evolution of inflammation in early stages of AD in relation to other pathological markers, such as deposition of amyloid plaques and tau as well as clinical presentation of the disease.



Similar content being viewed by others
References
Breitner JC, Gau BA, Welsh KA, Plassman BL, McDonald WM, Helms MJ et al (1994) Inverse association of anti-inflammatory treatments and Alzheimer’s disease: initial results of a co-twin control study. Neurology 44:227–232
Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG et al (2000) A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology 54:588–593
Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL et al (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289:2819–2826. doi:10.1001/jama.289.21.2819
Aisen PS, Davis KL (1994) Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am J Psychiatry 151:1105–1113
Rogers J, Webster S, Lue LF, Brachova L, Civin WH, Emmerling M et al (1996) Inflammation and Alzheimer’s disease pathogenesis. Neurobiol Aging 17:681–686
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. doi:10.1016/S1474-4422(15)70016-5
Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M (2014) Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans 42:1291–1301. doi:10.1042/BST20140107
Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A et al (2008) Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 451:720–724. doi:10.1038/nature06616
Boche D, Perry VH, Nicoll JA (2013) Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 39:3–18. doi:10.1111/nan.12011
Tang Y, Le W (2015) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. doi:10.1007/s12035-014-9070-5
Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R et al (2008) Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci 28:11650–11661. doi:10.1523/JNEUROSCI.3024-08.2008
Jacobs AH, Tavitian B (2012) Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab 32:1393–1415. doi:10.1038/jcbfm.2012.53
Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S et al (2009) Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol 35:306–328. doi:10.1111/j.1365-2990.2008.01006.x
Venneti S, Lopresti BJ, Wiley CA (2006) The peripheral benzodiazepine receptor (Translocator protein 18 kDa) in microglia: from pathology to imaging. Prog Neurobiol 80:308–322. doi:10.1016/j.pneurobio.2006.10.002
Venneti S, Wang G, Nguyen J, Wiley CA (2008) The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropathol Exp Neurol 67:1001–1010. doi:10.1097/NEN.0b013e318188b204
Levitt P, Pintar JE, Breakefield XO (1982) Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA 79:6385–6389
Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW (1985) Distinct monoamine oxidase A and B populations in primate brain. Science 230:181–183
Ekblom J, Jossan SS, Bergstrom M, Oreland L, Walum E, Aquilonius SM (1993) Monoamine oxidase-B in astrocytes. Glia 8:122–132. doi:10.1002/glia.440080208
Ekblom J, Jossan SS, Oreland L, Walum E, Aquilonius SM (1994) Reactive gliosis and monoamine oxidase B. J Neural Transm Suppl 41:253–258
Jossan SS, Ekblom J, Aquilonius SM, Oreland L (1994) Monoamine oxidase-B in motor cortex and spinal cord in amyotrophic lateral sclerosis studied by quantitative autoradiography. J Neural Transm Suppl 41:243–248
Jossan SS, Ekblom J, Gudjonsson O, Hagbarth KE, Aquilonius SM (1994) Double blind cross over trial with deprenyl in amyotrophic lateral sclerosis. J Neural Transm Suppl 41:237–241
Nakamura S, Kawamata T, Akiguchi I, Kameyama M, Nakamura N, Kimura H (1990) Expression of monoamine oxidase B activity in astrocytes of senile plaques. Acta Neuropathol 80:419–425
Saura J, Luque JM, Cesura AM, Da Prada M, Chan-Palay V, Huber G et al (1994) Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience 62:15–30
Jossan SS, Gillberg PG, Gottfries CG, Karlsson I, Oreland L (1991) Monoamine oxidase B in brains from patients with Alzheimer’s disease: a biochemical and autoradiographical study. Neuroscience 45:1–12
Gulyas B, Pavlova E, Kasa P, Gulya K, Bakota L, Varszegi S et al (2011) Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-l-deprenyl using whole hemisphere autoradiography. Neurochem Int 58:60–68. doi:10.1016/j.neuint.2010.10.013
Fowler JS, MacGregor RR, Wolf AP, Arnett CD, Dewey SL, Schlyer D et al (1987) Mapping human brain monoamine oxidase A and B with 11C-labeled suicide inactivators and PET. Science 235:481–485
Fowler JS, Volkow ND, Logan J, Schlyer DJ, MacGregor RR, Wang GJ et al (1993) Monoamine oxidase B (MAO B) inhibitor therapy in Parkinson’s disease: the degree and reversibility of human brain MAO B inhibition by Ro 19 6327. Neurology 43:1984–1992
Fowler JS, Wolf AP, MacGregor RR, Dewey SL, Logan J, Schlyer DJ et al (1988) Mechanistic positron emission tomography studies: demonstration of a deuterium isotope effect in the monoamine oxidase-catalyzed binding of [11C]l-deprenyl in living baboon brain. J Neurochem 51:1524–1534
Bergstrom M, Kumlien E, Lilja A, Tyrefors N, Westerberg G, Langstrom B (1998) Temporal lobe epilepsy visualized with PET with 11C-l-deuterium-deprenyl–analysis of kinetic data. Acta Neurol Scand 98:224–231
Kumlien E, Nilsson A, Hagberg G, Langstrom B, Bergstrom M (2001) PET with 11C-deuterium-deprenyl and 18F-FDG in focal epilepsy. Acta Neurol Scand 103:360–366
Engler H, Lundberg PO, Ekbom K, Nennesmo I, Nilsson A, Bergstrom M et al (2003) Multitracer study with positron emission tomography in Creutzfeldt–Jakob disease. Eur J Nucl Med Mol Imaging 30:85–95. doi:10.1007/s00259-002-1008-x
Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM et al (2007) Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci 255:17–22. doi:10.1016/j.jns.2007.01.057
Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R et al (1998) Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition. J Addict Dis 17:23–34. doi:10.1300/J069v17n01_03
Logan J, Fowler JS (2005) Evidence for reduced arterial plasma input, prolonged lung retention and reduced lung monoamine oxidase in smokers. Nucl Med Biol 32:521–529. doi:10.1016/j.nucmedbio.2005.03.004
Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE et al (2001) In-vivo measurement of activated microglia in dementia. Lancet 358:461–467. doi:10.1016/S0140-6736(01)05625-2
Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK et al (2008) Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiatry 64:835–841. doi:10.1016/j.biopsych.2008.04.021
Varrone A, Mattsson P, Forsberg A, Takano A, Nag S, Gulyas B et al (2013) In vivo imaging of the 18-kDa translocator protein (TSPO) with [18F]FEDAA1106 and PET does not show increased binding in Alzheimer’s disease patients. Eur J Nucl Med Mol Imaging 40:921–931. doi:10.1007/s00259-013-2359-1
Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N et al (2013) In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain 136:2228–2238. doi:10.1093/brain/awt145
Varrone A, Oikonen V, Forsberg A, Joutsa J, Takano A, Solin O et al (2015) Positron emission tomography imaging of the 18-kDa translocator protein (TSPO) with [18F]FEMPA in Alzheimer’s disease patients and control subjects. Eur J Nucl Med Mol Imaging 42:438–446. doi:10.1007/s00259-014-2955-8
Golla SS, Boellaard R, Oikonen V, Hoffmann A, van Berckel BN, Windhorst AD et al (2015) Quantification of [18F]DPA-714 binding in the human brain: initial studies in healthy controls and Alzheimer’s disease patients. J Cereb Blood Flow Metab 35:766–772. doi:10.1038/jcbfm.2014.261
Suridjan I, Pollock BG, Verhoeff NP, Voineskos AN, Chow T, Rusjan PM et al (2015) In-vivo imaging of grey and white matter neuroinflammation in Alzheimer’s disease: a positron emission tomography study with a novel radioligand, [F]-FEPPA. Mol Psychiatry. doi:10.1038/mp.2015.1
Pasqualetti G, Brooks DJ, Edison P (2015) The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep 15:17. doi:10.1007/s11910-015-0531-7
Varley J, Brooks DJ, Edison P (2014) Imaging neuroinflammation in Alzheimer’s and other dementias: recent advances and future directions. Alzheimers Dement. doi:10.1016/j.jalz.2014.08.105
Kropholler MA, Boellaard R, van Berckel BN, Schuitemaker A, Kloet RW, Lubberink MJ et al (2007) Evaluation of reference regions for (R)-[(11)C]PK11195 studies in Alzheimer’s disease and mild cognitive impairment. J Cereb Blood Flow Metab 27:1965–1974. doi:10.1038/sj.jcbfm.9600488
Tomasi G, Edison P, Bertoldo A, Roncaroli F, Singh P, Gerhard A et al (2008) Novel reference region model reveals increased microglial and reduced vascular binding of 11C-(R)-PK11195 in patients with Alzheimer’s disease. J Nucl Med 49:1249–1256. doi:10.2967/jnumed.108.050583
Yaqub M, van Berckel BN, Schuitemaker A, Hinz R, Turkheimer FE, Tomasi G et al (2012) Optimization of supervised cluster analysis for extracting reference tissue input curves in (R)-[(11)C]PK11195 brain PET studies. J Cereb Blood Flow Metab 32:1600–1608. doi:10.1038/jcbfm.2012.59
Kim S, Nho K, Risacher SL, Inlow M, Swaminathan S, Yoder KK et al (2013) Gene variation and microglial activity on [C]PBR28 PET in older adults at risk for Alzheimer’s disease. Multimodal Brain Image Anal 8159:150–158. doi:10.1007/978-3-319-02126-3_15
Versijpt JJ, Dumont F, Van Laere KJ, Decoo D, Santens P, Audenaert K et al (2003) Assessment of neuroinflammation and microglial activation in Alzheimer’s disease with radiolabelled PK11195 and single photon emission computed tomography. A pilot study. Eur Neurol 50:39–47
Gulyas B, Vas A, Toth M, Takano A, Varrone A, Cselenyi Z et al (2011) Age and disease related changes in the translocator protein (TSPO) system in the human brain: positron emission tomography measurements with [11C]vinpocetine. Neuroimage 56:1111–1121. doi:10.1016/j.neuroimage.2011.02.020
Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE et al (2008) Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis 32:412–419. doi:10.1016/j.nbd.2008.08.001
Yasuno F, Kosaka J, Ota M, Higuchi M, Ito H, Fujimura Y et al (2012) Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment-dementia converters measured by positron emission tomography with [(1)(1)C]DAA1106. Psychiatry Res 203:67–74. doi:10.1016/j.pscychresns.2011.08.013
Yokokura M, Mori N, Yagi S, Yoshikawa E, Kikuchi M, Yoshihara Y et al (2011) In vivo changes in microglial activation and amyloid deposits in brain regions with hypometabolism in Alzheimer’s disease. Eur J Nucl Med Mol Imaging 38:343–351. doi:10.1007/s00259-010-1612-0
Groom GN, Junck L, Foster NL, Frey KA, Kuhl DE (1995) PET of peripheral benzodiazepine binding sites in the microgliosis of Alzheimer’s disease. J Nucl Med 36:2207–2210
Wiley CA, Lopresti BJ, Venneti S, Price J, Klunk WE, DeKosky ST et al (2009) Carbon 11-labeled Pittsburgh Compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch Neurol 66:60–67. doi:10.1001/archneurol.2008.511
Schuitemaker A, Kropholler MA, Boellaard R, van der Flier WM, Kloet RW, van der Doef TF et al (2013) Microglial activation in Alzheimer’s disease: an (R)-[(1)(1)C]PK11195 positron emission tomography study. Neurobiol Aging 34:128–136. doi:10.1016/j.neurobiolaging.2012.04.021
Fan Z, Aman Y, Ahmed I, Chetelat G, Landeau B, Ray Chaudhuri K et al (2015) Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimers Dement 11(608–21):e7. doi:10.1016/j.jalz.2014.06.016
Lyoo CH, Ikawa M, Liow JS, Zoghbi SS, Morse CL, Pike VW et al (2015) Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with pet radioligand binding to translocator protein. J Nucl Med 56:701–706. doi:10.2967/jnumed.114.146027
McGeer EG, Singh EA, McGeer PL (1988) Peripheral-type benzodiazepine binding in Alzheimer disease. Alzheimer Dis Assoc Disord 2:331–336
Diorio D, Welner SA, Butterworth RF, Meaney MJ, Suranyi-Cadotte BE (1991) Peripheral benzodiazepine binding sites in Alzheimer’s disease frontal and temporal cortex. Neurobiol Aging 12:255–258
Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX (2006) Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience 138:749–756. doi:10.1016/j.neuroscience.2005.05.063
Gulyas B, Makkai B, Kasa P, Gulya K, Bakota L, Varszegi S et al (2009) A comparative autoradiography study in post mortem whole hemisphere human brain slices taken from Alzheimer patients and age-matched controls using two radiolabelled DAA1106 analogues with high affinity to the peripheral benzodiazepine receptor (PBR) system. Neurochem Int 54:28–36. doi:10.1016/j.neuint.2008.10.001
Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R et al (2014) Determination of [(11)C]PBR28 binding potential in vivo: a first human TSPO blocking study. J Cereb Blood Flow Metab 34:989–994. doi:10.1038/jcbfm.2014.46
Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R et al (2009) Microglial activation and amyloid deposition in mild cognitive impairment: a PET study. Neurology 72:56–62. doi:10.1212/01.wnl.0000338622.27876.0d
Marutle A, Gillberg PG, Bergfors A, Yu W, Ni R, Nennesmo I et al (2013) (3)H-deprenyl and (3)H-PIB autoradiography show different laminar distributions of astroglia and fibrillar beta-amyloid in Alzheimer brain. J Neuroinflammation 10:90. doi:10.1186/1742-2094-10-90
Hirvonen J, Kailajarvi M, Haltia T, Koskimies S, Nagren K, Virsu P et al (2009) Assessment of MAO-B occupancy in the brain with PET and [11C]-l-deprenyl-D2: a dose-finding study with a novel MAO-B inhibitor, EVT 301. Clin Pharmacol Ther 85:506–512. doi:10.1038/clpt.2008.241
Santillo AF, Gambini JP, Lannfelt L, Langstrom B, Ulla-Marja L, Kilander L et al (2011) In vivo imaging of astrocytosis in Alzheimer’s disease: an (1)(1)C-l-deuteriodeprenyl and PIB PET study. Eur J Nucl Med Mol Imaging 38:2202–2208. doi:10.1007/s00259-011-1895-9
Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B et al (2012) Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-l-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med 53:37–46. doi:10.2967/jnumed.110.087031
Choo IL, Carter SF, Scholl ML, Nordberg A (2014) Astrocytosis measured by (1)(1)C-deprenyl PET correlates with decrease in gray matter density in the parahippocampus of prodromal Alzheimer’s patients. Eur J Nucl Med Mol Imaging 41:2120–2126. doi:10.1007/s00259-014-2859-7
Nordberg A (2014) Molecular imaging in sporadic Alzheimer’s disease populations and those genetically at risk. Neurodegener Dis 13:160–162. doi:10.1159/000356333
Rodriguez-Vieitez E, Ni R, Gulyas B, Toth M, Haggkvist J, Halldin C et al (2015) Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: a correlative positron emission tomography and in vitro imaging study. Eur J Nucl Med Mol Imaging 42:1119–1132. doi:10.1007/s00259-015-3047-0
Acknowledgments
The work has been supported by funds from the Swedish Research Council (Project 05817), Karolinska Institutet Strategic Neuroscience program, the Stockholm County Council-Karolinska Institutet regional agreement on medical training and clinical research (ALF Grant), Swedish Brain Power, the Swedish Brain Foundation, the Alzheimer Foundation in Sweden, Karolinska Institutet’s Foundation for Aging Research, Swedish Foundation for Strategic Research (SFF), and by the EU project INMiND, FP7/2007-2013-no HEALTH-F2-2011-278850 (http://www.uni-muenster.de/InMind). Part of the work has been also supported by Bayer Healthcare, Berlin, Germany.
Contribution statement
Andrea Varrone is responsible for literature search and review, content planning, manuscript writing and editing. Agneta Nordberg contributed to literature search and review, content planning, manuscript writing and editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Andrea Varrone and Agneta Nordberg declare no conflicts of interest. The work performed using [18F]FEMPA has been supported by Bayer Healthcare, Berlin, Germany.
Human and animal studies
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study. In cases of animal studies, all institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Varrone, A., Nordberg, A. Molecular imaging of neuroinflammation in Alzheimer’s disease. Clin Transl Imaging 3, 437–447 (2015). https://doi.org/10.1007/s40336-015-0137-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-015-0137-8