Abstract
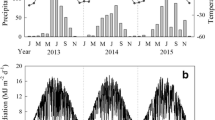
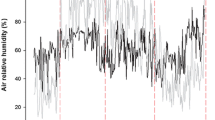
Forests and grasslands in arid and semi-arid regions receive high-intensity ultraviolet (UV) radiation year-round. However, how the UV radiation affects the litter decomposition on the forest floor remains unclear. Here, we conducted a field-based experiment in 2011 in the southeastern Horqin Sandy Land, Northeast China, to investigate the effects of UV radiation, litter layer thickness, and their interaction on the mass loss and chemical properties of decomposing litter from Xiaozhuan poplar (Populus × xiaozhuanica) and Mongolian pine (Pinus sylvestris var. mongolica) plantation trees. We found that UV radiation accelerated the decomposition rates of both the Xiaozhuan poplar litter and Mongolian pine litter. For both species, the thick-layered litter had a lower mass loss than the thin-layered litter. The interaction between UV radiation and litter layer thickness significantly affected the litter mass loss of both tree species. However, the effects of UV radiation on the chemical properties of decomposing litter differed between the two species, which may be attributed to the contrasting initial leaf litter chemical properties and morphology. UV radiation mostly had positive effects on the lignin concentration and lignin/N ratio of Xiaozhuan poplar litter, while it had negative effects on the N concentration of Mongolian pine litter. Moreover, litter layer thickness and its interaction with UV radiation showed mostly positive effects on the N concentration and lignin/N ratio of Xiaozhuan poplar litter and the ratios of C/N and lignin/N of Mongolian pine litter, and mostly negative effects on the C/N ratio of Xiaozhuan poplar litter and the N concentration of Mongolian pine litter. Together, these results reveal the important roles played by UV radiation and litter layer thickness in the process of litter decomposition in this semi-arid region, and highlight how changes in the litter layer thickness can exert strong influences on the photodegradation of litter in tree plantations.
Similar content being viewed by others
References
Aber J D, Melillo J M. 1982. Nitrogen immobilization in decaying hardwood leaf litter as a function of initial nitrogen and lignin content. Canadian Journal of Botany, 60(11): 2263–2269.
Adu-Bredu S, Yokota T, Ogawa K, et al. 1997. Tree size dependence of litter production, and above-ground net production in a young hinoki (Chamaecyparis obtusa) stand. Journal of Forest Research, 2(1): 31–37.
Aerts R. 1997. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos, 79(3): 439–449.
Austin A T, Vivanco L. 2006. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature, 442(7102): 555–558.
Austin A T, Ballaré C L. 2010. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proceedings of the National Academy of Sciences of the United States of America, 107(10): 4618–4622.
Berg B, Berg M P, Bottner P, et al. 1993. Litter mass loss rates in pine forests of Europe and Eastern United States: some relationships with climate and litter quality. Biogeochemistry, 20(3): 127–159.
Berg B, Johansson M B, Meentemeyer V. 2000. Litter decomposition in a transect of Norway spruce forests: substrate quality and climate control. Canadian Journal of Forest Research, 30(7): 1136–1147.
Brandt L A, King J Y, Milchunas D G. 2007. Effects of ultraviolet radiation on litter decomposition depend on precipitation and litter chemistry in a shortgrass steppe ecosystem. Global Change Biology, 13(10): 2193–2205.
Brandt L A, King J Y, Hobbie S E, et al. 2010. The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems, 13(5): 765–781.
Bremner J M. 1996. Nitrogen-total. In: Sparks D L, Page A L, Helmke P A, et al. Methods of Soil Analysis. Part 3. Chemical methods. Madison, Wisconsin: Soil Science Society of America, 1085–1122.
Caldwell M M, Bornman J F, Ballaré C L, et al. 2007. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochemical & Photobiological Sciences, 6(3): 252–266.
Chen F S, Zeng D H, Zhou B, et al. 2006. Seasonal variation in soil nitrogen availability under Mongolian pine plantations at the Keerqin Sand Lands, China. Journal of Arid Environments, 67(2): 226–239.
Cornwell W K, Cornelissen J H, Amatangelo K, et al. 2008. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters, 11(10): 1065–1071.
Coûteaux M M, Bottner P, Berg B. 1995. Litter decomposition, climate and liter quality. Trends in Ecology & Evolution, 10(2): 63–66.
Cox P M, Betts R A, Jones C D, et al. 2000. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature, 408(6809): 184–187.
Cybulski III W J, Peterjohn W T, Sullivan J H. 2000. The influence of elevated ultraviolet-B radiation (UV-B) on tissue quality and decomposition of loblolly pine (Pinus taeda L.) needles. Environmental and Experimental Botany, 44(3): 231–241.
Day T A, Zhang E T, Ruhland C T. 2007. Exposure to solar UV-B radiation accelerates mass and lignin loss of Larrea tridentata litter in the Sonoran Desert. Plant Ecology, 193(2): 185–194.
Day T A, Guénon R, Ruhland C T. 2015. Photodegradation of plant litter in the Sonoran Desert varies by litter type and age. Soil Biology and Biochemistry, 89: 109–122.
Foereid B, Bellarby J, Meier-Augenstein W, et al. 2010. Does light exposure make plant litter more degradable? Plant and Soil, 333(1–2): 275–285.
Gallo M E, Porras-Alfaro A, Odenbach K J, et al. 2009. Photoacceleration of plant litter decomposition in an arid environment. Soil Biology and Biochemistry, 41(7): 1433–1441.
Gaxiola A, Armesto J J. 2015. Understanding litter decomposition in semiarid ecosystems: linking leaf traits, UV exposure and rainfall variability. Frontiers in Plant Science, 6: 140.
Gehrke C, Johanson U, Callaghan T V, et al. 1995. The impact of enhanced ultraviolet-B radiation on litter quality and decomposition processes in Vaccinium leaves from the Subarctic. Oikos, 72(2): 213–222.
Hättenschwiler S, Tiunov A V, Scheu S. 2005. Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology, Evolution, and Systematics, 36: 191–218.
Hennessey T C, Dougherty P M, Cregg B M, et al. 1992. Annual variation in needle fall of a loblolly pine stand in relation to climate and stand density. Forest Ecology and Management, 51(4): 329–338.
Henry H A L, Brizgys K, Field C B. 2008. Litter decomposition in a California annual grassland: Interactions between photodegradation and litter layer thickness. Ecosystems, 11(4): 545–554.
Hoorens B, Aerts R, Stroetenga M. 2004. Elevated UV-B radiation has no effect on litter quality and decomposition of two dune grassland species: evidence from a long-term field experiment. Global Change Biology, 10(2): 200–208.
Iiyama K, Wallis A F A. 1990. Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. Journal of the Science of Food and Agriculture, 51(2): 145–161.
Killham K. 1994. Soil Ecology. New York: Cambridge University Press, 242.
King J Y, Brandt L A, Adair E C. 2012. Shedding light on plant litter decomposition: advances, implications and new directions in understanding the role of photodegradation. Biogeochemistry, 111(1–3): 57–81.
Lanzalunga O, Bietti M. 2000. Photo-and radiation chemical induced degradation of lignin model compounds. Journal of Photochemistry and Photobiology B: Biology, 56(2–3): 85–108.
Melillo J M, Aber J D, Muratore J F. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology, 63(3): 621–626.
Nelson D W, Sommers L E. 1996. Total carbon, organic carbon, and organic matter. In: Sparks D L, Page A L, Helmke P A, et al. Methods of Soil Analysis, Part 3: Chemical Methods. Madison, Wisconsin: Soil Science Society of America, 961–1010.
Newsham K K, Splatt P, Coward P A, et al. 2001. Negligible influence of elevated UV-B radiation on leaf litter quality of Quercus robur. Soil Biology and Biochemistry, 33(4–5): 659–665.
Pancotto V A, Sala O E, Cabello M, et al. 2003. Solar UV-B decreases decomposition in herbaceous plant litter in Tierra del Fuego, Argentina: potential role of an altered decomposer community. Global Change Biology, 9(10): 1465–1474.
Pancotto V A, Sala O E, Robson T M, et al. 2005. Direct and indirect effects of solar ultraviolet-B radiation on long-term decomposition. Global Change Biology, 11(11): 1982–1989.
Peng Q, Qi Y C, Dong Y S, et al. 2014. Decomposing litter and the C and N dynamics as affected by N additions in a semi-arid temperate steppe, Inner Mongolia of China. Journal of Arid Land, 6(4): 432–444.
Peterson E B, Peterson N M, Simard S W, et al. 1997. Paper birch managers' handbook for British Columbia. In: BC Ministry of Forests. FRDA Report 271. Victoria, Canada.
Rozema J, Tosserams M, Nelissen H J M, et al. 1997. Stratospheric ozone reduction and ecosystem processes: enhanced UV-B radiation affects chemical quality and decomposition of leaves of the dune grassland species calamagrostis epigeios. Plant Ecology, 128(1–2): 284–294.
Sayer E J. 2006. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biological Reviews, 81(1): 1–31.
Schimel J P, Bennett J. 2004. Nitrogen mineralization: challenges of a changing paradigm. Ecology, 85(3): 591–602.
Smith W K, Gao W, Steltzer H, et al. 2010. Moisture availability influences the effect of ultraviolet-B radiation on leaf litter decomposition. Global Change Biology, 16(1): 484–495.
Song X Z, Zhang H L, Chang S X, et al. 2012. Elevated UV-B radiation increased the decomposition of Cinnamomum camphora and Cyclobalanopsis glauca leaf litter in subtropical China. Journal of Soils and Sediments, 12(3): 307–311.
Song X Z, Peng C H, Jiang H, et al. 2013a. Direct and indirect effects of UV-B exposure on litter decomposition: a meta-analysis. PLoS ONE, 8(6): e68858.
Song X Z, Zhang H L, Jiang H, et al. 2013b. Influence of elevated UV-B radiation on leaf litter chemistry and subsequent decomposition in humid subtropical China. Journal of Soils and Sediments, 13(5): 846–853.
Song X Z, Jiang H, Zhang Z T, et al. 2014. Interactive effects of elevated UV-B radiation and N deposition on decomposition of Moso bamboo litter. Soil Biology and Biochemistry, 69: 11–16.
Throop H L, Archer S R. 2009. Resolving the dryland decomposition conundrum: some new perspectives on potential drivers. In: Lüttge U, Beyschlag W, Büdel B, et al. Progress in Botany. Berlin, Heidelberg: Springer, 171–194.
Uselman S M, Snyder K A, Blank R R, et al. 2011. UVB exposure does not accelerate rates of litter decomposition in a semi-arid riparian ecosystem. Soil Biology and Biochemistry, 43(6): 1254–1265.
Vitousek P M. 2004. Nutrient Cycling and Limitation: Hawaii as a Model System. Princeton, New Jersey, USA: Princeton University Press, 223.
Zeng D H, Hu Y L, Chang S X, et al. 2009. Land cover change effects on soil chemical and biological properties after planting Mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant and Soil, 317(1–2): 121–133.
Zepp R G, Erickson III D J, Paul N D, et al. 2007. Interactive effects of solar UV radiation and climate change on biogeochemical cycling. Photochemical & Photobiological Sciences, 6(3): 286–300.
Zhao L, Hu Y L, Lin G G, et al. 2013. Mixing effects of understory plant litter on decomposition and nutrient release of tree litter in two plantations in Northeast China. PLoS ONE, 8(10): e76334.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31270668, 41373038), the National Basic Research Program of China (2012CB416902) and the China Postdoctoral Science Foundation (2016M601342). We thank two anonymous reviewers for their valuable comments and suggestions. Special thanks are extended to LI Jingshi and AI Guiyan for their laboratory analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, B., Zhao, L., Zhao, Q. et al. Effects of ultraviolet (UV) radiation and litter layer thickness on litter decomposition of two tree species in a semi-arid site of Northeast China. J. Arid Land 10, 416–428 (2018). https://doi.org/10.1007/s40333-018-0054-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40333-018-0054-6