Abstract
Tobacco use is one of the major public health concerns and it is the most preventable cause of morbidity and mortality worldwide. Smoking cessation reduces subsequent cardiovascular events and mortality. Smoking is a real chronic disorder characterized by the development of an addiction status mainly due to nicotine. This condition makes the smokers generally unable to quit smoking without help. Different strategies are available to treat smoking dependence that include both non-pharmacological (behavioral counselling) and pharmacological therapies. Currently, it is well accepted that smoking cessation drugs are effective and safe in real-world settings. Nicotine replacement therapy (NRT), varenicline, bupropion and cytisine are the main pharmacological strategies available for smoking cessation. Their efficacy and safety have been proved even in patients with chronic cardiovascular disease. Each of these drugs has peculiar characteristics and the clinician should customize the smoking cessation strategy based on currently available scientific evidence and patient's preference, paying particular attention to those patients having specific cardiovascular and psychiatric comorbidities. The present document aims to summarize the current viable pharmacological strategies for smoking cessation, also discussing the controversial issue regarding the use of alternative tobacco products, in order to provide useful practical indications to all physicians, mainly to those involved in cardiovascular prevention.
Similar content being viewed by others
1 Introduction
Tobacco use is one of the major public health concerns and it is the most preventable cause of morbidity and mortality worldwide. It is estimated that 16 million adults are currently living with a smoking-related disease in the United States [1]. Cigarette smoking is the main source of tobacco consumption and it is also the most dangerous one. Every year, more than 700,000 people die because of smoke-related diseases in Europe. More than one-quarter of Europeans smoke and one-third of smokers are 25–39 years of age [2]. There are nearly 12 million smokers in Italy, mostly men, although female smokers increased by over a million in 2016 [3].
It is known that smokers’ life expectancy is on average 10 years shorter than non-smokers’. Moreover, half of the smokers lose about 20 years of healthy life before dying because of a smoke-related disease [4]. Besides being involved in the pathophysiology of several types of malignant neoplasms, not only in the lungs, smoking represents the first cause of chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD) [5, 6], as well as a major determinant of cardiovascular (CV) diseases, such as myocardial infarction, stroke and peripheral arterial diseases. In patients with hypertension, despite optimal blood pressure control, smoking was independently associated with early markers of atherosclerosis [7].
Regarding CV risk, 10–30% of all CV deaths worldwide are related to cigarette smoking. Experimental epidemiological and clinical studies have demonstrated a non-linear trend between smoke amount and CV effects, so even a low amount of smoke is associated with a disproportionately high CV risk [8]. This non-linear trend explains why non-smokers exposed to passive smoking have a 25–30% increased CV risk and why it is important to warn all patients with heart disease to avoid second-hand smoke (SHS) [4].
Smoking cessation reduces subsequent CV events and mortality [9]. Regardless the duration and intensity of smoking habit, comorbidities or age, all smokers may take advantage by quitting smoking, even if cessation happens after the development of a CV disease [8]. Indeed, quitting smoking after a major cardiovascular event is likely the most effective secondary prevention measure [3], particularly in COPD patients, where it reduces both the risk of worsening chronic respiratory disease and the recurrence of CV events at the same time [9].
Cigarette smoking is also related to a higher susceptibility to pulmonary bacterial and viral infections, inducing structural changes in the respiratory tract and a decrease in immune response. In fact previous studies have shown that smokers are twice more likely to contract influenza and have more severe symptoms than non-smokers [1]. Moreover, in the current era of a viral pandemic due to the Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) causing the 2019 coronavirus disease (COVID-19), despite the limited data available, smoking has been assumed to be possibly associated with adverse prognosis. A recent study has demonstrated that ever-smokers (current and former) have an upregulated pulmonary angiotensin-converting-enzyme-2 (ACE2) gene expression, thus leading to an increased risk for viral binding and entry of both SARS-CoV and SARS-CoV-2 in their lungs [10]. This panorama warrants a compelling, prompt, and effective attempt for smoking cessation.
The present document aims to inform the overall population about the risks correlated to smoking as well as to provide useful practical indications to all physicians, mainly to those involved in CV prevention, about the current viable pharmacological strategies for smoking cessation.
2 Definitions and Tobacco Products
Smoking is a real chronic disorder related to the intermittent and compulsive consumption of an exogenous substance, sustained by the development of an addiction status, which is mainly due to nicotine [11]. “Addiction” is defined as the individual perception of a constraint of smoking with struggles to stop or reduce smoking for long intervals of time. The practice is continued despite harmful consequences and due to the occurrence of withdrawal symptoms when the exposure to nicotine ends [8]. A “smoker” is an individual who currently smokes tobacco every day and who has smoked at least 100 cigarettes in his/her entire life [2].
In the last decade, we witnessed the evolution of many tobacco products; at present, we can divide all these different products into two main categories:
-
tobacco combustion products, in which tobacco burns producing smoke inhaled by the user (i.e. cigarettes). Inhalation of combustion products exposes the organism to a wider spectrum of harmful chemical substances, causing a greater risk for health compared to tobacco products that do not use combustion (i.e. snuff, chewing, dipping tobacco), even though no form of tobacco is entirely risk-free;
-
alternative products without tobacco combustion, mainly represented by electronic devices that deliver nicotine, including electronic cigarettes (e-cigarettes), and other devices that heat tobacco instead of burning it (heat-not-burn, HNB). An e-cigarette heats a liquid containing nicotine, propylene glycol and/or vegetable glycerine, and flavored chemical substances in order to produce vapors inhaled by the user. Since the e-cigarette does not burn tobacco, it does not generate combustion smoke, but only vapors. In HNB products, the tobacco is heated to a lower temperature than a combusted cigarette to create aerosols, instead of smokes, inhaled by the user.
2.1 Nicotine Addiction
Smokers are generally unable to quit smoking without help, mainly because they develop a tobacco addiction. This condition represents a real nosological entity and not just a bad habit. The main determinant of tobacco addiction is nicotine [12]. Nicotine addiction is a chronic, relapsing disorder that needs a long-term management and an intensive treatment approach, like other chronic diseases. Nicotine addiction has two components: physical dependence and psychological dependence [11, 13].
The main feature of nicotine addiction is the desire to feel the pharmacological effects of nicotine and the avoidance of possible withdrawal symptoms. Indeed, nicotine is responsible for both positive, such as psychoactive stimulation, and negative effects, such as discomfort and uneasiness [13].
The mechanism responsible for addiction development and the resulting chronic and reiterated use has its base in the pharmacodynamics of nicotine. Nicotine binds specific acetylcholine receptors, mostly neuronal nicotinic acetylcholine receptors (nAChRs) α4β2 subtypes, in the central nervous system, and stimulates the release of neuromodulators (mainly dopamine) promoting pleasure and thus perpetuating consumption [13].
The degree of such addiction can be quantified through simple and validated screening tools, such as the Fagerström Test for Nicotine Dependence (FTND) [14] (see Table 1) or the Heaviness of Smoking Index (HSI) [15]. The scores obtained from these tests can predict both the likelihood of starting tobacco consumption again after stopping ("relapse") and the selection of the most beneficial therapeutic strategy to quit smoking.
Epidemiological data showed that nicotine addiction is a chronic recurrent condition acquired primarily during adolescence [16]. "Relapse" means start again to smoke regularly after cessation. Among former smokers, relapse is common and occurs during the first days following the attempt of discontinuing smoking, when withdrawal symptoms are generally more severe. More than 75% of people who try to quit smoking by themselves relapse within the first week, making this time-period critical [17]. Nevertheless, the risk of relapse is quite common even in smokers who suspended smoking for longer periods. Patients who are free from smoking for at least 12 months have a likelihood of relapse of 35% during their life [17]. Nicotine addiction is characterized by a specific withdrawal syndrome, just like other pathological dependence syndromes. Nicotine withdrawal syndrome develops in the first 4–12 h following smoking cessation. The main symptoms include craving for smoking, irritability/aggressiveness/rage, anxiety, restlessness, fatigue, increased appetite, difficulty concentrating, depression, headache, insomnia, dizziness/vertigo [12]. The set of symptoms could vary from an individual to another. All of these manifestations are temporary and they reach the greatest intensity in the first 24–72 h, decreasing in the following 3–4 weeks. Symptoms usually persist for more than 3–4 weeks in 40% of patients [12].
3 How to Quit Smoking: The “5 A’s” and the “5 R’s” Models
Five steps are usually needed to treat tobacco use and dependence in the clinical setting, known as the “5 A’s” model [18].
-
Ask about tobacco use. Identify and document tobacco use status for every patient at every visit.
-
Advise quitting. In a clear, strong and personalized manner, urge every tobacco user to quit at every visit.
-
Assess willingness to make a quit attempt. Is the tobacco user willing to make a quit attempt at this time?
-
Assist in quit attempt. For the patient willing to make a quit attempt, offer medication and provide or refer for counselling or additional treatment to help the patient quit.
-
Arrange follow-up. For the patient willing to make a quit attempt, arrange follow-up contacts, beginning within the first week after the quit date and then for the next 12 months, managing the risk of relapse or the relapse itself. For patients unwilling to make a quit attempt at the time, address tobacco dependence and willingness to quit at the next clinic visit.
For smokers willing to quit at the time of the visit, it is recommended to provide immediately integrated support (pharmacological and behavioral). For patients unwilling to quit at the time of the visit, it is advised to implement interventions designed to increase future quit attempts.
In order to increase future quit attempts, the clinician should follow the "5 R's" model [18].
-
Relevance of quitting to patient. The patient should answer the question "why it is so important to quit smoking?"
-
Risks for the patient's health if he/she continues tobacco use.
-
Rewards of quitting explaining the benefits for patient's health.
-
Roadblocks to quitting, to understand what possibly could prevent the achievement of smoking cessation.
-
Repetition of the discussion relative to the attempt of quitting, regardless of the patient's will to quit at the time of the conversation.
4 Pharmacological Approach to Smoking Cessation
Different pharmacological strategies are available to treat smoking dependence [19]. They should be associated with non-pharmacological strategies (behavioral counselling), which are validated approaches to promote the process of quitting.
The main pharmacological treatments available for smoking cessation are the following: nicotine replacement therapy (NRT), varenicline, bupropion and cytisine (see Table 2) [19].
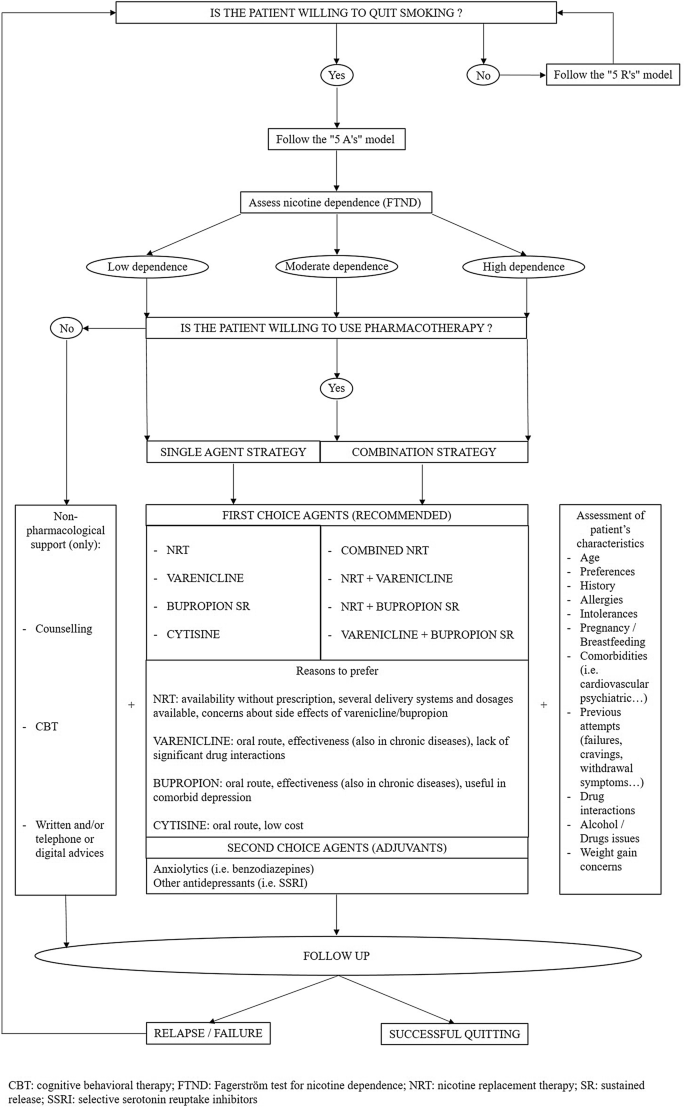
The clinician should follow both currently available scientific evidence and patient's preference for a proper choice of one therapy over another, paying particular attention to those patients having contraindications to these drugs due to the presence of specific comorbidities (i.e. CV, renal, hepatic, psychiatric comorbidities) (see Fig. 1) [12].
4.1 Nicotine Replacement Therapy (NRT)
Nicotine replacement therapy is based on the controlled administration of nicotine, making it easier to abstain from tobacco by partially replacing the nicotine previously obtained from tobacco use. Nicotine replacement therapy aims both to stimulate nicotine receptors thus removing smoking craving and withdrawal symptoms (this effect is immediate), and to reduce the number of nicotine receptors (this effect is slower and continues for weeks progressively reducing tobacco dependence).
Controlled administration of nicotine reduces the positive effects induced by smoking thanks to a lower dosage and slower pharmacokinetics. Nicotine is more slowly absorbed generating lower but prolonged blood peaks, compared to cigarettes, thus reducing rewarding effects and withdrawal symptoms including irritability, anxiety, difficulty concentrating, dysphoria, increased appetite, weight gain, and sleep disorders [12]. Each different NRT product has demonstrated the same efficacy in achieving smoking cessation. Consequently, the choice of the NRT product should reflect the patient's preferences. Transdermal nicotine patch is commonly the first choice because it yields higher compliance rates than other NRT products [8].
Due to a more beneficial pharmacokinetic profile, combination NRT (short plus long-acting products) is superior to single NRT. A combination of transdermal nicotine patch with a more rapidly absorbed NRT (i.e. oro-nasal products) is more effective than the use of a single product [8, 20, 21]. Combination NRT is the current standard of therapy, once the clinician decides to use NRT as therapeutical strategy.
Transdermal nicotine patches deliver nicotine at a relatively steady rate (“controller” NRTs), so they are the most suitable routes of administration to reduce withdrawal symptoms. On the other hand, chewing gums, lozenges, inhalers and nasal sprays (“reliever” NRTs) reduce symptoms faster than patches, but they provide worse basal coverage [12].
It is important to note that uncontrolled use of nicotine inhalers or sprays could determine high blood peaks of nicotine, resembling what happens with traditional cigarettes, thus providing some of the contentment associated with smoking and hence slowing down the process of cessation [12].
Oral formulations contain nicotine absorbed by the oral mucosa. The main weakness of this class is the strict dosing scheme needed, sometimes with a fixed short interval of 1–2 h. Moreover, nicotine is absorbed by the oral mucosa only with a neutral mouth's pH. Therefore, the patient should be advised to avoid meals or drinks during or in the 15 min before drug use, paying attention to avoid mostly acid foods and beverages. Rather, inhalers have the advantage of mimicking cigarette usage, preserving the hand-to-mouth action of smoking [12].
Nicotine replacement therapy usually lasts 12 weeks, although treating heavy smokers for longer intervals may be reasonable, at least until the patient feels confident enough not to relapse. Long-term NRT is not associated with increased incidence of harm. Longer therapeutic intervals are particularly indicated in patients with psychiatric or other substance use disorders [12].
There are no contraindications to nicotine substitutes except evidence of allergy, which is rare for patients using patches and unusual for patients using oral formulations. In some countries, they are contraindicated during pregnancy.
Controlled, longitudinal, and case-controlled trials with NRT in patients with CV diseases did not report greater risks of adverse events compared to placebo. It has been found an increase in CV symptoms, such as tachycardia and arrhythmia, expected because of the sympathomimetic effects of nicotine, but no increase in major CV events (death, myocardial infarction, stroke) [8].
Nicotine could potentially worsen CV diseases through his sympathomimetic properties. However, nicotine levels in NRT are generally lower compared to those in tobacco products. Moreover, NRT does not expose users to the harmful products of combustion, which are major determinants in the pathogenesis of CV diseases. In conclusion, albeit NRT is not probably harmless, it is surely way less damaging than cigarette smoking.
Typical adverse events common to all NRT products include gastrointestinal symptoms (nausea, vomiting, abdominal pain, diarrhea), dizziness, headache, local irritation according to the route of administration. Adverse event due to NRT could be managed by adjusting the dosage to minimize symptoms or, alternatively, by changing molecule [12].
Patients usually interpret some symptoms as adverse events due to treatment, instead of symptoms related to smoking cessation. Depression and sleep disorders are the most common withdrawal symptoms mistaken for adverse events to treatment. Many individuals who quit smoking show more or less severe signs and symptoms of depression.
Depression symptoms are not linked to the therapy itself but rather to the presence of a latent depression, unmasked by smoking cessation. Patients with a prior history of depression should be followed carefully to avoid a relapse of depression symptoms. Patients who are currently experiencing depression should start an antidepressant treatment, as indicated by current guidelines, simultaneously with strategies for smoking cessation [12].
When craving persists, there is no risk of overtreatment with NRTs. Conversely, in a patient who is not experiencing any desire for smoking, an overdose induces the impression of excessive smoking, associated with signs like nausea and tachycardia. These signs are transient and vanish quickly after treatment suspension. Therefore, it is possible to reintroduce the therapy at a lower dose. On the other hand, typical signs of underdosing are craving, irritability, anxiety, food craving with frequent snacks, insomnia and smoking despite NRT [19].
4.2 Varenicline
Varenicline is a partial agonist selective for α4β2 nicotinic acetylcholine receptors (nAChRs), one of the receptors related to dopamine release following nicotine binding. Varenicline activates the α4β2 receptor with a maximal effect about 50% that of nicotine, alleviating symptoms of craving and withdrawal (agonist activity), while simultaneously reducing the rewarding by preventing nicotine binding (antagonist activity). Ultimately, varenicline promotes smoking cessation by preventing withdrawal symptoms while moderate levels of dopamine are maintained in the brain [19].
Several meta-analyses suggest that varenicline is as effective as combination NRT. These two approaches are considered first-line therapy for smoking cessation, especially in patients with CV disease [22, 23].
Smokers should stop smoking one to two weeks after the first dose of varenicline, in order to reach the steady state. The pre-medication phase may be prolonged to 4 weeks to increase efficacy [24].
The drug is progressively titrated to minimize side effects, mainly gastrointestinal symptoms such as nausea (see Table 2). Varenicline is substantially excreted by the kidney. Dose reduction is needed in patients with severe renal impairment (estimated glomerular filtration rate below 30 ml/min). In these patients, the maximum recommended dose is 1 mg once daily. Varenicline is not recommended in patients with end-stage renal disease, due to the lack of data in this population. Patients who cannot tolerate adverse reactions of varenicline may lower the dose temporarily or permanently. Nausea could be reduced assuming varenicline with food or reducing the dose to 0.5 mg twice a day. Sleep disorders, particularly vivid dreams, could be avoided progressively reducing the evening dose until suspension, maintaining the morning dose only.
An additional course of 12 weeks treatment with varenicline at 1 mg twice daily may be considered for the maintenance of abstinence in patients who have successfully stopped smoking at the end of the therapeutic interval (12 weeks) [25].
Absolute or relative contraindications to varenicline use are hypersensitivity to the active substance or any of the excipients, age < 18 years old, pregnancy and breastfeeding [12]. Varenicline has no clinically meaningful drug interactions. Safety studies on varenicline in patients with stable CV disease found no increase in CV events, even though no univocal data are available in patients with acute coronary syndrome. Therefore, varenicline could be used safely in patients with stable CV disease and with caution in patients with acute coronary syndrome [26].
Soon after the introduction of varenicline, reports of an association with depression and suicidal ideation arose. A review of 11 published studies suggest that such association is likely to be weak [23]. Previous studies compared smokers having a previous psychiatric history (anxiety, depression, psychotic or bipolar disorder) with a control group demonstrating that treatment with varenicline was not associated with a higher risk of psychiatric adverse events [27]. Nevertheless, the possibility warrants patients' monitoring for clinical and therapeutical evaluation if neuropsychiatric symptoms occur, including behavioral changes, hostility, agitation, depressive mood, suicidal ideation.
The most common adverse events are nausea and sleep disorders. In the majority of cases, nausea is mild to moderate in intensity, occurs early in the treatment period, lasting on average for 12 days, and seldom results in discontinuation. Dosing titration is likely to reduce the incidence of nausea. Patients should be informed that this symptom usually solves within one week after starting treatment and could be avoided taking the drug with food [12]. Other side effects have been reported such as abdominal pain, constipation, abdominal distension, abnormal dreams, insomnia, dizziness, dry mouth, weight increase, increased appetite, and headache. These symptoms developed twice more frequently with varenicline than placebo [28].
In the majority of cases, they occur in the first 4 weeks of treatment, are mild to moderate in severity and temporary. Varenicline may influence the ability to drive. Previous reports found higher incidence of car accidents among patients taking varenicline. However, subsequent studies did not confirm this correlation [29].
4.3 Bupropion, Sustained-Release (SR)
The precise mechanism of action of bupropion is not well understood. Bupropion is likely to exert its pharmacological effects by weakly inhibiting the reuptake of both dopamine and norepinephrine, therefore prolonging their duration of action within the neuronal synapse and their downstream effects. When taken to quit smoking, bupropion may confer both anti-craving and anti-withdrawal effects by inhibiting dopamine reuptake, which mediates the reward pathways associated to nicotine use, and through the antagonism of the nicotinic acetylcholinergic receptor [30].
Bupropion also acts by alleviating some of the symptoms of nicotine withdrawal, which include depression, reducing the overall severity of withdrawal syndrome. Highly nicotine-dependent smokers who receive bupropion are more likely to experience a decrease in depressive symptoms associated with abstinence. Nevertheless, bupropion efficacy for nicotine addiction is due to a distinct property other than its anti-depressant activity. In fact, its positive outcomes regarding smoking cessation have been demonstrated even in patients not suffering from depression [31].
Bupropion nearly doubled smoking cessation rates compared to placebo, and it was equally effective in men and women [31].
Bupropion is recommended also to avoid weight increase after smoking cessation. Indeed, Hays et al. reported both better weight control and higher smoking cessation rates than placebo one year after bupropion discontinuation [30]. Furthermore, it could be useful to prevent relapse both in smokers and in alcoholic patients. Patients who quitted smoking using bupropion for 7 weeks delayed relapse if they continued taking it for a total of 52 weeks. In COPD patients, bupropion could impair ventilatory responses to hypoxia and hypercapnia, leading to potentially harmful effects on disease progression. However, no studies on COPD patients taking bupropion confirmed such hypothesis [32].
Bupropion should be started before the patient’s planned quit day. The patient should set a “target quit date” within the first 2 weeks of therapy and could continue smoking during treatment since this does not significantly affect the pharmacodynamics of bupropion. Steady-state blood levels are usually achieved after 1–2 weeks of treatment. If the patient is not able to quit within the target date, it is possible to delay smoking suspension until the third or fourth week of treatment or when abstinence is reached [12].
The recommended and maximum dosage of bupropion is 300 mg daily (150 mg twice daily). The dosage could be decreased to 150 mg daily if the patient does not tolerate the entire dose due to adverse reactions occurrence. Two previous RCTs found similar efficacy between bupropion 300 mg daily and 150 mg daily doses, but the latter was associated with fewer side effects [33].
Bupropion should be used with caution in patients with liver disease and renal disease. The dose recommended in these patients is 150 mg daily.
Absolute or relative contraindication to bupropion use are pregnancy and breastfeeding, history of seizures, epilepsy, brain and cranium neoplasms, current or prior diagnosis of bulimia or anorexia nervosa, simultaneous discontinuation of alcohol or sedatives/benzodiazepines, current use of monoaminoxydase inhibitors or use of these drugs in the previous 14 days [12].
Regarding patients with CV disease, no clinical study highlighted any safety concern [34]. Insomnia, dry mouth, and headache are the most common adverse events associated with bupropion use. Insomnia is certainly the most frequent adverse event and it could be avoided by taking the first dose of bupropion early in the morning and the second dose in the late afternoon, at least 4 h prior bedtime. Insomnia could be limited even reducing the dose to 150 mg daily [12].
A very small risk of seizure exists, and it is the most alarming adverse event associated with bupropion. It is quite rare (1:1000) and it is facilitated by some pre-existing risk factors, such as severe head injury, epilepsy, food disorders, arteriovenous malformations, cerebral neoplasms or infections, severe stroke and cerebrovascular disease, concomitant use of other medications that lower the seizure threshold [12]. Rare cases of angioedema [35] and syndrome of inappropriate antidiuretic hormone secretion have been reported, with the latter frequently associated with antipsychotic therapy [36].
4.4 Cytisine
Cytisine is a natural alkaloid found in several plant genera, such as Cytisus Laburnum and Sophora Tetraptera. Cytisine acts similarly to varenicline. It is a partial agonist selective for α4β2 nicotinic acetylcholine receptors, responsible for nicotine effects, and it prevents nicotine binding, thus reducing rewarding and both withdrawal symptoms and craving. Cytisine is available as oral tablets containing 1.5 mg of active principle [37].
Clinical data on cytisine found an efficacy similar, or even higher, than NRT regarding the likelihood of smoking cessation. However, cytisine has a greater propensity to adverse events, even though they are mainly minor such as nausea, vomiting, and sleep disorders [38, 39].
The recommended dose is 1 tablet (1.5 mg) every 2 h up to 6 tablets per day on day 1–3. In the meantime, the patient should reduce smoking to avoid nicotine overdosing symptoms. If the desired therapeutic effect is not obtained, the treatment should be interrupted and another cycle could be attempted after 2–3 months. If indeed a good response is achieved, the patient continues the treatment with 5 tablets per day (1 tablet every 2.5 h) on day 4–12, suspending smoking on day 5. The following therapy proceeds with 4 tablets per day (1 tablet every 3 h) on day 13–16, then with 3 tablets per day (1 tablet every 5 h) on day 17–20, lastly with 1 or 2 tablets per day (1 tablet every 6–8 h) on day 21–25 [19].
Italian experience reports a longer treatment schedule (up to 40 days), which begins with a progressive increase in dosage of cytisine 1.5 mg. The maximum dose of 6 tablets per day is gradually reached in 7 days. Target quit date should be set between the 8th and the 14th day. Then the dose decreases slowly until the 40th day [40]. Cytisine overdosing is similar to nicotine intoxication and produces nausea, vomiting, clonic seizures, tachycardia, mydriasis, headache, fatigue, respiratory pump failure.
4.5 Anxiolytics and Other Antidepressants
Many smokers are also affected by anxiety and depressive disorders, which could be emphasized by nicotine deprivation and/or the loss of smoking habit. Almost every smoker who attempts to quit manifests withdrawal symptoms such as irritability, restlessness, psychomotor agitation, insomnia, and anxiety. Although there are no specific data regarding the use of anxiolytics for smoking cessation, their role remains essential to manage associated anxiety disorders. Anxiolytics such as benzodiazepines (i.e. low dose and sustained-release alprazolam), could be helpful for the management of withdrawal symptoms [41]. Specific treatment is indicated if an overt mixed anxiety-depressive disorder is present, together with the smoking cessation drug regimens.
The most effective and safe antidepressants are selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), atypical antidepressants (i.e. mirtazapine), and serotonin modulators (i.e. trazodone). Due to their proven effectiveness and tolerability, and the amount of available evidence, SSRI should be the first choice. Among SSRIs, sertraline and escitalopram have a good efficacy/safety profile [42].
5 Therapeutic Efficacy and Combination Therapy
Currently, it is well accepted that smoking cessation drugs are effective in real-world settings and in different conditions coexisting with smoking habit (i.e. other substances abuse, depression, anxiety) [43]. Previous studies found that treatments for smoking cessation doubled abstinence rates after 6 months compared to placebo. Combination NRT and varenicline were associated with higher abstinence rates (33% and 37%, respectively) [43]. It is important to note that several studies took into account also psychological and behavioural counselling. Following studies confirmed that combination NRT and varenicline had comparable efficacy and both of them had higher efficacy than single NRT or bupropion [43].
Drug therapy for smoking cessation typically lasts 12 weeks. However, these strategies could be personalised based on the clinician and/or patient's experience. Nicotine replacement therapy could last longer and even indefinitely, if needed. Indeed, the Food and Drug Administration (FDA) approved NRT for more than 3 months since it does not produce significant risks. Similarly, varenicline use can be extended for an additional 3-month period to prevent relapses. Indeed, varenicline was found to be effective and safe if administered for 24 weeks [25].
Long-term use of bupropion is possible, extending the treatment for further 7–9 weeks [44]. If bupropion is used to treat a depressive disorder, therapeutic changes or suspension should take into account also the possible relapse of depressive symptoms.
If we consider pharmacodynamics, it is possible to speculate that combination therapy with different drugs having complementary modes of action should achieve better outcomes. Indeed, the FDA approved both the combination NRT and the combination of single NRT and bupropion for smoking cessation [20].
The Italian Society of Tobaccology (Società Italiana di Tabaccologia—SITAB) guidelines suggest the following combination therapies: long-term use of nicotine patches (more than 14 weeks) plus a short-term NRT product (gum or spray) or nicotine patches plus bupropion SR. There is no evidence of contraindications for the use of NRT plus varenicline, but results on efficacy are not univocal. Further research is needed to support the efficacy of this approach in clinical practice. In conclusion, all treatments including NRT, varenicline and bupropion are effective for smoking cessation. Their efficacy and safety have been proved even in patients with chronic CV disease. Combination therapies are justified for patients with a major nicotine addiction or who have used monotherapies which have failed because of relapses [40].
5.1 Focus on Smokers with CV Disease
Several meta-analyses showed that both varenicline and combination NRT are more effective than bupropion or single NRT in smokers suffering from a chronic CV disease [4], making the first two approaches the first-line therapies for smoking cessation in patients with a history of CV disease (see Table 3). Few evidence is available in patients with acute coronary syndromes, especially regarding safety concerns. Based on the current state of the art, it is advisable to start a therapeutic approach once the CV condition has been stabilized. A progressive strategy could be helpful to improve compliance, since drug regimens after an acute coronary syndrome may be complex. Therefore, it is allowed to introduce further drug therapies gradually. Given the shorter latency for efficacy and the greater ease of use, it is generally advised to start with NRT rather than varenicline or bupropion, since the latter ones need an imbrication period while the patient is still smoking [4].
6 Alternative Tobacco Products
In the last years, several alternatives to traditional cigarettes have spread, such as electronic cigarettes (e-cigarettes) and heat-not-burn (HNB) devices. The marketing claims the potential of these products as an approach for smoking cessation. However, there is no definitive evidence supporting this claim, with the majority of the studies run by the same manufacturing companies instead of independent parties. E-cigarette may be helpful in suspending conventional smoking [45]. However, 80% of former smokers continued using e-cigarettes after quitting traditional smoking, thus remaining nicotine-addicted. On the other hand, 14 out of 15 longitudinal real-life studies demonstrated how e-cigarettes use undermined abstinence significantly, with 60–80% of patients continuing to smoke conventional cigarettes [4]. Regarding HNB products, there is no evidence supporting their efficacy and the use of concomitant traditional cigarettes is highly prevalent.
Another matter of debate is the supposed harmlessness of these alternative products. Almost all independent studies underlined potential harm. It is well-established that e-cigarettes' liquids vaporization and aerosolization may produce harmful substances such as benzene and formaldehyde.
Studies on animals have highlighted the onset of endothelial cells dysfunction and the induction of oxidative stress damages. Human studies showed the emergence of airways obstruction, the deregulation of normal pulmonary homeostasis, and the increase in chronic asthma exacerbations even in the short-term [4].
Furthermore, HNB products effects on human health are even more obscure. Data on animals found pro-inflammatory effects and data on humans found no improvement in pulmonary function after switching from combusted to heated tobacco. Independent studies found that HNB products are likely to have the pathogenic potential for COPD development [46].
The advent of alternative tobacco products generated a great appeal on new generations, thus largely increasing e-cigarette use among younger adults in both Europe and US. Indeed, e-cigarettes have become the most commonly used tobacco product among young people. This "epidemic" highlighted two aspects. The first one has a major impact on the short-term and it is the occurrence of increasingly acute respiratory syndromes, widely described in the scientific literature [47]. The second aspect has major consequences in the long-term and it represents the risk of switching back from alternative to conventional smoking [4].
A recent study conducted in Italy showed that almost all e-cigarette users among Italian smokers consumed liquids containing nicotine and most of them used these devices in indoor environments where conventional cigarette smoking is usually banned [48]. Consequently, to avoid the dual use (i.e. concomitant use of electronic and conventional cigarettes), we support the recent suggestion made by the World Health Organization (WHO) to forbid e-cigarettes, at least in public places and workplaces where conventional smoking is already prohibited.
At present, there is no sufficient evidence to adequately evaluate health hazards associated with alternative products use. Furthermore, there is no sufficient evidence regarding alternative products efficacy to support their use for smoking cessation. There are in-vivo data and clinical reports describing the clinical harm of using alternative tobacco products [49].
Therefore, taking into account the scarce evidence sustaining the use of alternative tobacco products, health workers should not recommend them as a treatment strategy for smoking cessation [49]. Moreover, these alternative devices may be harmful, even if they have been considered less dangerous than conventional smoking in the beginning.
7 Role of Air Pollution on CV Health
Air pollution is formed by the combination of particulate matter (PM), mainly derived from the combustion of carbonaceous particles, and several gaseous pollutants, such as nitrogen dioxide, nitric oxide, sulphur dioxide, carbon monoxide, volatile organic compounds and ozone. The size of PM determines its classification into coarse (PM10), fine (PM2.5) and ultrafine particles [50].
Epidemiological studies found that exposure to air pollution increases both long-term and short-term CV mortality through an increased incidence of major CV events [50]. Pathophysiological studies found acute functional consequences of air pollution exposure in both myocardial and pulmonary blood flow regulation, but also in coagulation function, mainly driven by the sharp increase in reactive oxygen species generation, which impairs vasodilatation mediated by nitric oxide and promotes vascular inflammation [50]. Indeed, short-term exposure to PM2.5, even at low concentrations within current air quality standards, was associated with significant blood pressure increase in high CV risk patients [51]. Although both the level and duration of exposure are directly associated with increased CV risk, there is no safe threshold below which there is no effect, and concentrations of fine and especially ultrafine particles in ambient air are thought to be strongly underestimated by actual standards and measurements [50]. Therefore, alongside the efforts to reduce the burden of CV diseases related to smoking, the promotion of safer air quality is going to be the next challenge in CV disease prevention, as recognized by the European Society of Cardiology’s campaign “Environment & the Heart”.
8 Conclusion
Smoking reduces the quantity and above all the quality of life. Smoking cessation strategies should be implemented in every clinical context, particularly in those involved in CV risk management and prevention. The pharmacological approach to smoking cessation is safe and effective, and therefore should be offered to everyone willing to quit smoking. It is never too late to quit smoking, and we should be able to make morbidity and mortality related to smoking a thing of the past.
References
Adams JM. Smoking cessation - progress, barriers, and new opportunities: the surgeon general's report on smoking cessation. JAMA. 2020. https://doi.org/10.1001/jama.2020.6647.
Ward BWCT, Nugent CN, Schiller JS. Early release of selected estimates based on data from the 2015 National Health Interview Survey. Hyattsville: National Center for Health Statistics; 2016.
Volpe M, Battistoni A, Gallo G, Rubattu S, Tocci G, Writing C, et al. Executive summary of the 2018 joint consensus document on Cardiovascular Disease Prevention in Italy. High Blood Pressure Cardiovasc Prevent Off J Italian Soc Hypertens. 2018;25(3):327–41.
Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332–65.
Collaborators USBoD, Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, et al. The state of US Health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444–722.
Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–17.
Giudice R, Izzo R, Manzi MV, Pagnano G, Santoro M, Rao MA, et al. Lifestyle-related risk factors, smoking status and cardiovascular disease. High Blood Pressure Cardiovasc Prevent Off J Italian Soc Hypertens. 2012;19(2):85–92.
Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519.
Ferri C. Strategies for reducing the risk of cardiovascular disease in patients with chronic obstructive pulmonary disease. High Blood Pressure Cardiovasc Prevent Off J Italian Soc Hypertens. 2015;22(2):103–11.
Cai G, Bossé Y, Xiao F, Kheradmand F, Amos CI. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;201(12):1557–9.
Jarvis MJ. Why people smoke. BMJ. 2004;328(7434):277–9.
U.S. Department of Health and Human Services. Smoking Cessation. A report of the surgeon general. In: Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf. Accessed 19 June 2020.
Picciotto MR, Kenny PJ. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harbor Perspect Med. 2013;3(1):a012112.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–27.
Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–9.
Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 1990;9(6):701–16.
Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med. 2003;18(12):1053–7.
World Health Organization. Toolkit for delivering the 5A’s and 5R’s brief tobacco interventions in primary care. 2014. https://www.who.int/tobacco/publications/smoking_cessation/9789241506953/en/. Accessed 5 Jun 2020.
European Network for Smoking and Tobacco Prevention. ENSP Guidelines for treating tobacco dependence the comprehensive guide to the implementation of treatments and strategies to treat tobacco dependence. 2016. https://elearning-ensp.eu/assets/English%2520version.pdf. Accessed 5 Jun 2020.
Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–62.
Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169(22):2148–55.
Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med. 2012;44(6):588–97.
Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Datab Syst Rev. 2013;5:CD009329.
Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171(8):770–7.
Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR, et al. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):64–71.
Sterling LH, Windle SB, Filion KB, Touma L, Eisenberg MJ. Varenicline and adverse cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5(2):e002849.
McClure JB, Swan GE, Catz SL, Jack L, Javitz H, McAfee T, et al. Smoking outcome by psychiatric history after behavioral and varenicline treatment. J Subst Abuse Treat. 2010;38(4):394–402.
Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: a placebo-controlled, randomized study. Respirology. 2009;14(3):384–92.
Molero Y, Lichtenstein P, Zetterqvist J, Gumpert CH, Fazel S. Varenicline and risk of psychiatric conditions, suicidal behaviour, criminal offending, and transport accidents and offences: population based cohort study. BMJ. 2015;2(350):h2388.
Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Ann Internal Med. 2001;135(6):423–33.
Hughes JR. Depression during tobacco abstinence. Nicotine Tobacco Res Off J Soc Res Nicotine Tobacco. 2007;9(4):443–6.
Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet. 2001;357(9268):1571–5.
Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med. 2003;163(19):2337–444.
Benowitz NL, Pipe A, West R, Hays JT, Tonstad S, McRae T, et al. Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Internal Med. 2018;178(5):622–31.
Tackett AE, Smith KM. Bupropion-induced angioedema. Am J Health-System Pharm AJHP Off J Am Soc Health-System Pharm. 2008;65(17):1627–30.
Kim CS, Choi JS, Bae EH, Kim SW. Hyponatremia associated with bupropion. Electrolyte Blood Pressure E & BP. 2011;9(1):23–6.
Tutka P, Zatonski W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacol Rep PR. 2006;58(6):777–98.
Walker N, Howe C, Glover M, McRobbie H, Barnes J, Nosa V, et al. Cytisine versus nicotine for smoking cessation. N Engl J Med. 2014;371(25):2353–62.
West R, Zatonski W, Cedzynska M, Lewandowska D, Pazik J, Aveyard P, et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med. 2011;365(13):1193–200.
Società Italiana di Tabaccologia (SITAB). Linee guida per il trattamento della dipendenza da tabacco 2018. https://www.tabaccologia.it/filedirectory/PDF/guidelines_2018_online.pdf. Accessed 5 Jun 2020.
Hughes JR, Stead LF, Lancaster T. Anxiolytics for smoking cessation. Cochrane Datab Syst Rev. 2000;4:CD002849.
Howes S, Hartmann-Boyce J, Livingstone-Banks J, Hong B, Lindson N. Antidepressants for smoking cessation. Cochrane Datab Syst Rev. 2020;4:CD000031.
Phs Guideline Update Panel L, Staff. Treating tobacco use and dependence: 2008 update US Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53(9):1217–22.
Wilkes S. The use of bupropion SR in cigarette smoking cessation. Int J Chronic Obstruct Pulmonary Disease. 2008;3(1):45–53.
Glantz SA, Bareham DW. E-cigarettes: use, effects on smoking, risks, and policy implications. Annu Rev Public Health. 2018;1(39):215–35.
Ratajczak A, Jankowski P, Strus P, Feleszko W. Heat not burn tobacco product—a new global trend: impact of heat-not-burn tobacco products on public health, a systematic review. Int J Environ Res Public Health. 2020;17(2):409.
Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, et al. Pulmonary illness related to e-cigarette use in illinois and Wisconsin—final report. N Engl J Med. 2020;382(10):903–16.
Gallus S, Borroni E, Liu X, Carrozzi L, Dalla Pietra G, Eslami Varzaneh S, et al. Electronic cigarette use among Italian smokers: patterns, settings, and adverse events. Tumori. 2020;26:300891620915784.
Pisinger C, Dagli E, Filippidis FT, Hedman L, Janson C, Loukides S, et al. ERS and tobacco harm reduction. Eur Respir J. 2019;54(6):1902009.
Bourdrel T, Bind MA, Bejot Y, Morel O, Argacha JF. Cardiovascular effects of air pollution. Arch Cardiovasc Diseases. 2017;110(11):634–42.
Giorgini P, Rubenfire M, Das R, Gracik T, Wang L, Morishita M, et al. Particulate matter air pollution and ambient temperature: opposing effects on blood pressure in high-risk cardiac patients. J Hypertens. 2015;33(10):2032–8.
Acknowledgements
Unconditional support for article publication charges was provided by Novartis Farma S.p.A.
Funding
This research received no specific Grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Giulietti, F., Filipponi, A., Rosettani, G. et al. Pharmacological Approach to Smoking Cessation: An Updated Review for Daily Clinical Practice. High Blood Press Cardiovasc Prev 27, 349–362 (2020). https://doi.org/10.1007/s40292-020-00396-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-020-00396-9