Abstract
Objectives
Pembrolizumab monotherapy showed significantly longer overall survival and fewer treatment-related adverse events compared to chemotherapy in patients with advanced or metastatic non-small cell lung cancer (NSCLC) with programmed death ligand-1 (PD-L1)–positive tumors in the first-line setting in KEYNOTE (KN)-024 and in those previously treated in KN010. The objective of this analysis was to assess the benefit–risk of pembrolizumab in terms of quality-adjusted survival amongst patients in these trials.
Methods
The Quality-adjusted Time Without Symptoms of disease progression or Toxicity of treatment (Q-TWiST) analysis was used to compare treatments. Survival time was partitioned into three health states: with toxicity before disease progression, without toxicity before disease progression, and disease progression until death. Health state utilities were estimated using EuroQol-5 Dimensions, 3 Levels (EQ-5D-3L) data collected in the trials. Q-TWiST was calculated as the utility-weighted sum of the mean health state durations. Trial data analyzed included the primary analysis and subsequent data cutoffs. The base-case analysis was based on the most recent analysis of the trials.
Results
Patients randomized to pembrolizumab had 2.49 months greater Q-TWiST (P value < 0.001) compared to those randomized to platinum-based chemotherapy at a follow-up of 24 months in KN024, and 2.29 months greater Q-TWiST (P value < 0.001) compared to docetaxel over 30 months follow-up in KN010. Results across the trial analyses showed an increase in trend for the Q-TWiST improvement of pembrolizumab over time.
Conclusions
Pembrolizumab showed significant improvement in Q-TWiST compared to chemotherapy in advanced or metastatic NSCLC in both previously untreated and treated patients. The benefits of pembrolizumab continued to accrue with longer follow-ups.
Similar content being viewed by others
As a novel regimen for the treatment of advance or metastatic non-small cell lung cancer (NSCLC), pembrolizumab monotherapy has been shown to substantially prolong patient survival without added burden of treatment-related toxicity in patients whose tumors express programmed death ligand-1 (PD-L1). |
This is the first Quality-adjusted Time Without Symptoms of disease progression or Toxicity of treatment (Q-TWiST) analysis to quantify the benefit–risk of pembrolizumab by concurrently evaluating survival time, disease progression, safety profile and quality of life across the clinical trials. |
Pembrolizumab had statistically significant and clinically meaningful improvement in quality-adjusted survival based on the Q-TWiST analysis compared to chemotherapy in PD-L1–positive advanced or metastatic NSCLC. |
1 Introduction
Lung cancer, of which 85–90% is classified as non-small cell lung cancer (NSCLC), continues to be a leading cause of death worldwide [1]. Until recently, chemotherapy was the standard treatment for advanced or metastatic NSCLC, which offered limited efficacy but significant toxicity [2]. Recent development in immunotherapy has increased treatment options for NSCLC patients [3]. As a highly selective, humanized, IgG4 monoclonal antibody against programmed death ligand-1 (PD-L1), pembrolizumab has shown significant clinical benefit for the treatment of metastatic NSCLC in several randomized trials. For example, pembrolizumab demonstrated significantly longer overall survival (OS) compared to chemotherapy as a first-line treatment in patients whose tumors have high PD-L1 expression [tumor proportion score (TPS) ≥ 50%] [4, 5] and in those whose tumors express PD-L1 (TPS ≥ 1%) [6]. Additionally, for second-line advanced NSCLC treatment, pembrolizumab showed significant improvement in OS compared to docetaxel in patients whose tumors express PD-L1 (TPS ≥ 1%) [7, 8]. In these trials, treatment-related adverse events (AEs) were less common with pembrolizumab compared to the chemotherapy arm.
To complement the efficacy and safety data from clinical trials, the Quality-adjusted Time Without Symptoms of disease progression or Toxicity of treatment (Q-TWiST) analysis can be used to evaluate the overall effect of treatment interventions. Q-TWiST, which is categorized as a health index in the classification of benefit–risk methods described by Mt-Isa et al. [9], compares treatments by evaluating both quantity and quality of survival time using a single metric [10]. It defines specific health states related to disease progression and toxicities and assigns quality-of-life (QoL) utility weights to each health state. It then compares treatments based on the overall utility-weighted survival time.
Q-TWiST builds on the quality-adjusted life year (QALY), which is a well-established metric used by health economists for conducting cost-effectiveness analyses. Previous health economic analyses have assessed the incremental QALYs and demonstrated the cost-effectiveness of pembrolizumab compared to chemotherapy in previously treated and untreated advanced and metastatic NSCLC [11, 12]. However, QALYs are usually derived from extrapolation of the survival outcomes from the trial period to a patient’s life time, and therefore, are often associated with a high level of uncertainty. Q-TWiST evaluates quality-adjusted life time in a clinical trial setting, thus avoiding the need for extrapolation, and can serve as another useful method to inform decision making for healthcare providers and regulatory agencies.
Recently, the American Society of Clinical Oncology (ASCO) Value Framework [13] proposed metrics that combine clinical benefit, side effects, and improvement in patient symptoms or QoL. Q-TWiST provides a comprehensive clinical assessment to compare net clinical benefits of therapies in a clinical trial setting by integrating patient preferences (i.e., utilities) into the analysis. It provides insights into clinical benefit–risk of treatment selection, especially from a patient perspective. There is an increasing interest among payors and regulators [14, 15] in making patient-centered assessments. Originally developed for evaluating breast cancer treatments [16,17,18], the Q-TWiST method has been used to evaluate clinical benefits and risks in many different cancer indications in the past few years [19,20,21,22]. Thus, the aim of this study was to compare the benefit–risk of pembrolizumab to that of chemotherapy in patients with metastatic NSCLC based on the clinical trials using the Q-TWiST methodology.
2 Methods
2.1 Data Source
The data sources for this analysis were the KEYNOTE-024 and KEYNOTE-010 trials that evaluated pembrolizumab versus standard of care chemotherapy.
KEYNOTE-024 (KN024) [4, 5]: KN024 was an international, randomized, open-label, phase 3 trial, which compared pembrolizumab with standard-of-care platinum-based chemotherapy as first-line therapy for patients with metastatic NSCLC that expresses high levels of PD-L1 (defined as TPS ≥ 50%) .
The trial included patients aged 18 years or older with stage IV NSCLC, no sensitizing EGFR mutations, and no ALK translocations, who were naïve to systemic therapy for metastatic disease. Patients were randomly assigned, in a 1:1 ratio, to receive treatment with either pembrolizumab (administered intravenously at a dosage of 200 mg every 3 weeks) or the investigator’s choice of one of five platinum-based chemotherapy regimens.
Treatment was continued for the specified number of cycles or until disease progression, intolerable toxicity, patient withdrawal, or investigator decision. Patients in the chemotherapy group who had disease progression, could cross over to receive pembrolizumab if safety criteria were met. Patients in either treatment group who were in a clinically stable condition and were considered by the investigator to be deriving clinical benefit could continue therapy after disease progression.
The primary endpoint was progression-free survival (PFS), which was defined as the time from randomization to disease progression or death. PFS was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 [23], by means of blinded, independent, central radiologic review (BICR). Secondary endpoints included OS, defined as the time from randomization to death from any cause, objective response rate and safety. Health state utility profiles assessed using the EuroQol-5 Dimensions, 3 Levels (EQ-5D-3L) measure was an exploratory endpoint. In addition, it was also a pre-defined exploratory objective to evaluate Q-TWiST within each treatment arm in the trial.
The Q-TWiST analysis was based on the intent-to-treat (ITT) population in the trial. Data from final analysis based on the most recent cutoff date of July 10, 2017 with median follow-up of 25.2 months (range 20.4–33.7 months) [5] were used in the base-case analysis. Data from interim analysis 2 with a cutoff date of May 9, 2016 and median duration of follow-up of 11.2 months (range 6.3–19.7 months) [4] were also examined as a sensitivity analysis.
KEYNOTE-010 (KN010) [7, 8]: KN010 was an international, randomized, open-label, phase 2/3 trial, which compared pembrolizumab with docetaxel for patients with previously treated, PD-L1–positive (TPS ≥ 1%), advanced NSCLC.
The trial included patients aged 18 years or older with advanced NSCLC, with progression after two or more cycles of platinum-doublet chemotherapy. Patients were randomly assigned, in a 1:1:1 ratio, to receive pembrolizumab 2 mg/kg or 10 mg/kg intravenously every 3 weeks (for 35 cycles), or docetaxel 75 mg/m2 intravenously every 3 weeks.
Treatment was continued for 24 months or until disease progression, intolerable toxic effects, physician decision, patient withdrawal, or other reasons. Patients who progressed according to investigator assessment could remain on treatment until a confirmatory scan done 4–6 weeks later. Per protocol, patients in the docetaxel group were not permitted to cross over to receive pembrolizumab.
The primary trial endpoints were PFS and OS. Secondary endpoints included response and safety. In addition, health status was assessed using the EQ-5D-3L, a preference-based measure of health, which was an exploratory endpoint.
The ITT population in the pembrolizumab 2 mg/kg [as this is the dosing approved through the Food and Drug Administration (FDA)/European Medicines Agency (EMA) labels] and docetaxel groups was included in this analysis. Data from the most recent cutoff date of March 24, 2017 with a median follow-up of 31 months (range 23–41 months) [8] and data from the cutoff date of September 30, 2015 with a median follow-up of 13.1 months (range 5.7–23.7 months) [7] were examined in this analysis as base-case and sensitivity analyses, respectively.
2.2 Statistical Analysis
2.2.1 Definition of Health States
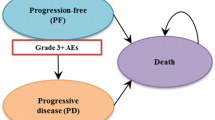
We applied the standard Q-TWiST methodology, wherein patient survival time was partitioned into three health states:
-
1.
Toxicity (TOX): time with grade 3 + AEs after randomization and before disease progression.
Grading of AEs was based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0 [24]. The time spent with all-cause grade 3 + AEs before disease progression was summed for each patient, and a day with multiple events was only counted once. All grade 3 + AEs were included in the analysis, apart from those starting before randomization or after progression. Time spent with toxicities unresolved by progression was truncated at the date of progression. Patients who did not experience grade 3 + AEs before disease progression were assigned a duration of zero for the TOX state. Patients with censored PFS also had TOX time censored.
-
2.
Time without symptoms or toxicities (TWiST): time from randomization to disease progression without grade 3 + AEs.
The TWiST state was defined as PFS time minus time with TOX. Patients who were alive and had no disease progression or who were lost to follow-up were censored at the time of the last tumor assessment.
-
3.
Relapse (REL): time from disease progression to death.
The REL state was defined as OS minus PFS time. Patients who were alive or who were lost to follow-up were censored at the time of the last contact.
2.2.2 Estimate of QoL Utility Weights
Health state utilities were derived based on EQ-5D-3L data collected in both the KN024 and KN010 trials.
In the KN024 trial, the EQ-5D questionnaire was administered on day 1 of treatment cycles 1, 2, 3, 6, 9, and 12 and every third cycle thereafter as long as patients were on study treatment, as well as at the treatment discontinuation visit and at a 30-day post-treatment safety follow-up visit.
In the KN010 trial, the EQ-5D-3L was administered on day 1 of treatment cycles 1, 2, 3, 5, 9, and 13 and every fourth treatment cycle thereafter as long as patients were on study treatment, as well as at the treatment discontinuation visit and 30-day post-treatment safety follow-up visit.
Patient responses to the EQ-5D-3L were converted to population-based utility values using published algorithms. Different scoring functions by nationality were used in the analysis. Specifically, US-based scores were applied for US patients [25], UK-based scores for UK patients [26] and EU-based scores for all other patients [27]. The EQ-5D scores were averaged by the health state represented on the day of completing the measure for the overall trial population.
2.2.3 Calculation of Q-TWiST and Evaluation of Treatment Effects
Survival curves that corresponded to TOX, PFS and OS were estimated by the Kaplan–Meier (KM) method. The restricted mean duration of each health state was derived from the area under the KM curve. The mean durations of TWiST and REL were calculated as follows:
The mean Q-TWiST represents QoL-adjusted mean OS, which was calculated as follows:
where TOX, TWiST, and REL represent the mean health state durations and UTOX, UTWiST and UREL denote the average utility weight for each health state.
In this analysis, the mean Q-TWiST for each treatment arm and the difference in means between treatment arms were calculated. The 95% confidence intervals (CIs) for the mean differences and the P values were estimated using T test based on the normal approximation, in which variance was estimated by bootstrapping with 25,000 replications [28]. Additionally, the relative gains in Q-TWiST, defined as the mean absolute Q-TWiST differences divided by the mean OS of the chemotherapy arm, were calculated.
For each data cutoff, two different time horizons were analyzed. The primary analyses were restricted to the median overall follow-up time. This is typically done to ensure robustness of Q-TWiST analyses. This restriction may, however, lead to a conservative estimate of the benefit for immuno-oncology therapies, as they often show delayed treatment effects. Therefore, secondary analyses were conducted over the longest survival follow-up to explore the treatment difference over extended follow-up time. In addition, the mean Q-TWiST difference was calculated and plotted graphically to illustrate the treatment benefit of pembrolizumab as a function of follow-up time in the two trials.
Revicki et al. [29] recommended that the clinically important difference for Q-TWiST is 10% of OS in a study, and that differences of 15% are clearly clinically important. This criterion was used to assess the clinical importance of the Q-TWiST improvement of pembrolizumab in this analysis.
2.3 Sensitivity Analysis
Both KN024 and KN010 have included the primary analysis and all subsequent data cutoffs from the respective trials. To examine the impact of data cutoff and follow-up duration on the clinical effect of pembrolizumab, a sensitivity analysis was conducted using the first published analysis of KN024 (protocol specified interim analysis 2 with a data cutoff date of May 9, 2016; median duration of follow-up 11.2 months) and KN010 (protocol specified final analysis with a data cutoff date of September 30, 2015; median follow-up 13.1 months).
Sensitivity analysis was also conducted to incorporate lower grade AEs. The Q-TWiST was re-evaluated with grade 2 AEs added in the TOX state.
Additional analyses were conducted to explore the effect of differing utility values on the Q-TWiST results. First, the standardized utility values of UTOX = 0.5, UTWiST = 1.0, and UREL = 0.5 were used in the sensitivity analysis, which was the most common set used historically when there was no instrument for utility measurement implemented in the clinical trials. The coefficient of TWiST (UTWiST) was assigned 1 to represent a reference state against which the two other states could be compared. Second, a panel of sensitivity analyses were conducted on a full range of UTOX and UREL (from 0 to 1) to assess the robustness of the treatment benefit with respect to uncertainty in utility values.
3 Results
The KN024 trial enrolled 305 patients between September 19, 2014, and October 29, 2015, with 154 randomized to pembrolizumab and 151 to chemotherapy. The ITT population included all randomized patients and was included in this analysis. Patients were well balanced across treatment groups and a complete listing of patient characteristics was published in the clinical paper [4]. As of the data cutoff date, July 10, 2017, 94 patients (62.3%) in the chemotherapy group crossed over to receive pembrolizumab or other anti–programmed cell death protein 1 (anti–PD-1)/anti–PD-L1 therapies after discontinuation of the study drug. This could potentially diminish survival benefit of pembrolizumab. Patients in the pembrolizumab group had superior OS compared with patients in the chemotherapy group (HR 0.63, 95% CI 0.47–0.86). The proportion of patients experiencing any grade 3 + AEs was 61.7% for pembrolizumab and 74.7% for platinum-based chemotherapy [5].
In the KN010 trial, 1034 patients were enrolled in the study between August 28, 2013, and February 27, 2015: 345 were allocated to pembrolizumab 2 mg/kg, 346 to pembrolizumab 10 mg/kg, and 343 to docetaxel [7].
As of the data cutoff date of March 24, 2017, patients in the pembrolizumab 2 mg/kg group had superior OS (hazard ratio HR = 0.73, 95% CI 0.62–0.87) compared with patients in the docetaxel group. The proportion of patients experiencing a grade 3 + AE was 49.3% for pembrolizumab and 56.6% for docetaxel [8].
3.1 Duration of the Health States
Partitioned survival plots based on KN024 are shown separately for pembrolizumab and chemotherapy in Figure 1. The area between the curves illustrates the time in each of the three health states in the Q-TWiST calculation.
Given the median follow-up in the KN024 trial of 25.2 months, the primary analysis was restricted to the 24 months of follow-up. An additional analysis was conducted using data up to 30 months, which is close to the longest survival follow-up. Table 3 summarizes the mean duration in each health state by treatment group for both primary and additional analyses. The primary results suggested that patients randomized to pembrolizumab had 0.76 months longer (P = 0.205) TOX, 4.12 months longer (P < 0.001) TWiST and 1.91 months shorter REL (P = 0.004) compared to those in the chemotherapy group. Compared with the primary analysis, the additional analysis resulted in the same duration of TOX and TWiST, but the duration of REL was extended for both arms, and the difference in REL between treatments was smaller (mean 0.82 months, P = 0.288).
Partitioned survival plots of KN010 are shown in Fig. 2. The primary analysis was restricted to the 30 months of follow-up given the median follow-up of 31 months of the trial. An additional analysis was conducted using data up to 40 months. Both analyses yielded the result of significantly longer TWiST (P < 0.001) with pembrolizumab compared to docetaxel. The mean duration in each health state by treatment group for both primary and additional analyses was summarized in Table 4.
3.2 Utility Weights
QoL utility weights for each health state are summarized in Tables 1 and 2. The mean utility score for treatment-naïve NSCLC patients was estimated from the EQ-5D data in KN024, and the score was 0.727 in TOX, 0.803 in TWiST and 0.716 in REL. Based on KN010, previously treated patients had mean utility scores of 0.710, 0.775 and 0.688 in TOX, TWiST and REL states, respectively. Consistent with expectations, the utility scores calculated based on the EQ-5D-3L were highest in the TWiST state and lowest in REL. The difference in mean utility scores between TOX and REL state was not statistically significant.
3.3 Q-TWiST
The results demonstrated that patients in the pembrolizumab group had a significantly longer Q-TWiST compared to those receiving chemotherapy in both KN024 and KN010, as shown in Tables 3 and 4.
The Q-TWiST difference favored pembrolizumab by 2.49 months (P < 0.001) with 24 months of follow-up in KN024, with relative gain in Q-TWiST equivalent to 18% of OS time (given the restricted mean OS of 14.07 months in the chemotherapy group) in the population of treatment-naïve metastatic NSCLC. The extended Q-TWiST was 3.27 months after 30 months, with relative Q-TWiST gain of 20% of mean OS time in this population.
In KN010, the Q-TWiST difference was 2.29 months (P < 0.001; relative gain of 20%) favoring pembrolizumab with 30 months of follow-up, and 3.12 months (P < 0.001; relative gain of 25%) after 40 months.
As shown in Figs. 3 and 4, the Q-TWiST gain of pembrolizumab consistently increased over the extended follow-up time in both trials.
Mean Q-TWiST gain of pembrolizumab vs platinum-based chemotherapy over time in TPS ≥ 50% treatment-naïve metastatic NSCLC patients in KN024, based on data cutoff of July 10, 2017. KN024 KEYNOTE-024, NSCLC non-small cell lung cancer, TPS tumor proportion score, Q-TWiST Quality-adjusted Time Without Symptoms of disease progression or Toxicity of treatment
Mean Q-TWiST gain of pembrolizumab versus docetaxel over time in TPS ≥ 1% previously treated advanced NSCLC patients in KN010, based on data cutoff of March 24, 2017. KN010 KEYNOTE-010, NSCLC non-small cell lung cancer, TPS tumor proportion score, Q-TWiST Quality-adjusted Time Without Symptoms of disease progression or Toxicity of treatment
3.4 Sensitivity Analysis
The sensitivity analysis showed significantly longer Q-TWiST for pembrolizumab compared to chemotherapy based on the first published analysis of both KN024 (with a data cutoff of May 9, 2016) and KN010 (with a data cutoff of September 30, 2015), although the magnitude of improvement is smaller than the base-case results due to the shorter follow-up. The Q-TWiST difference in KN024 was 0.92 months (P < 0.001) with 12 months of follow-up and 1.47 months (P < 0.001) at 18 months. In KN010, the Q-TWiST difference was 0.54 months (P = 0.002), favoring pembrolizumab with 12 months of follow-up, and 1.45 months (P < 0.001) at 21 months.
The analysis that included grade 2 + AEs in the TOX state also resulted in significant Q-TWiST gain of pembrolizumab in both trials. The mean utility score was 0.760 for TOX and 0.882 for TWiST in KN024 and 0.745 for TOX and 0.792 for TWiST in KN010. The mean Q-TWiST difference was 2.43 months (P < 0.001; relative gain of 17%) over 24 months of follow-up in KN024 and 2.29 months (P < 0.001; relative gain of 20%) at 30 months in KN010.
Applying the standardized utility values of UTOX = 0.5, UTWiST = 1.0, and UREL = 0.5 resulted in mean Q-TWiST differences of 3.54 months (P < 0.001; relative gain of 25%) at a 24-month follow-up in KN024 and 2.29 months (P < 0.001; relative gain of 20%) at 30 months in KN010. The Q-TWiST gains at median follow-up ranged from 2.21 to 4.87 months (relative Q-TWiST gain from 16% to 35%) in KN024, and 1.45 to 3.13 months (relative Q-TWiST gain from 13% to 27%) in KN010 as UTOX and UREL vary from 0 to 1.
4 Discussion
As a novel regimen for the treatment of metastatic NSCLC, pembrolizumab monotherapy has been shown to substantially prolong patient survival without added burden of treatment-related toxicity in patients whose tumors express PD-L1. This is the first Q-TWiST analysis to quantify the benefit–risk of pembrolizumab by concurrently evaluating survival time, disease progression, safety profile and QoL across the clinical trials. Q-TWiST is a protocol pre-specified exploratory endpoint in the KN024 trial; however, it is a post hoc analysis for the KN010.
The results demonstrated significant Q-TWiST improvement of pembrolizumab in both trials in all scenarios. The magnitude of the improvement increased with longer follow-up time. Our findings demonstrated a highly robust benefit–risk associated with pembrolizumab in PD-L1–positive metastatic NSCLC.
In the primary analysis of KN024, the significantly longer gain in the Q-TWiST of 2.49 months associated with pembrolizumab compared to platinum doublet chemotherapy in previously untreated metastatic NSCLC was 18% of the mean survival time of this population, a statistically significant benefit which exceeded the ‘clearly clinically important’ benchmark of 15% suggested by Revicki et al. [29]. Similarly, the primary results in the Q-TWiST analysis of KN010 demonstrated that pembrolizumab exceeded the published criteria for a ‘clearly clinically important’ improvement in previously treated advanced NSCLC.
However, Revicki et al. also suggested that “it is unknown whether observations based on HRQL or health preference studies also generalize to Q-TWiST studies” and that caution should be exercised when interpreting criteria of the clinical significance of Q-TWiST results. We therefore further evaluated this analysis in the context of historical Q-TWiST studies in oncology. Based on a recent systematic review [30] of 81 Q-TWiST comparisons across 51 articles, mean absolute Q-TWiST gain and relative Q-TWiST gain across 22 immunotherapy analyses was reported as 2.07 months and 8.9%, respectively. The relative Q-TWiST gains at median follow-up using standardized utilities for KN024 and KN010 are larger than the relative Q-TWiST gains of about 95% of immunotherapy studies reported in the review. Such Q-TWiST gains are higher when longer, full follow-up is analyzed.
Our findings also enable an understanding of the nature of the benefit–risk associated with pembrolizumab in PD-L1–positive metastatic NSCLC. The Q-TWiST improvement with pembrolizumab in KN024 was found to be almost entirely accounted for by increased duration of TWiST health state. The duration of REL was slightly shorter in the pembrolizumab group compared to chemotherapy, as a large proportion of patients in the chemotherapy arm crossed over to receive pembrolizumab or other immune-oncology drugs and had a prolonged post-progression survival [31]. This was anticipated to diminish the survival benefit of pembrolizumab. In KN010, the improvement in Q-TWiST with pembrolizumab was accounted for by both increased duration of pre- and post-progression survival.
To better understand the impact of AEs on the Q-TWiST results, sensitivity analysis was conducted to incorporate grade 2 AEs. Results showed that inclusion of grade 2 AEs had only a negligible impact on mean Q-TWiST estimate in both treatment arms in the two trials. The absolute and relative Q-TWiST gains were consistent with the base-case results.
Strengths of this study are that analyses are presented using both patient QoL utility directly assessed during the trials, and also using standardized utility weights. Patient QoL utilities were directly assessed during the trials by the inclusion of the EQ-5D-3L questionnaire. The derived utility scores were significantly lower during both the TOX and REL health states than during the TWiST health state. The average deterioration of QoL was larger upon disease progression than the deterioration due to grade 3 + AEs as observed in both trials. One limitation of the Q-TWiST analysis, however, is that utility weights are assigned by health state but not by treatment. As evaluated in previous utility analyses [32, 33], patients in the pembrolizumab group had higher utility scores in all health states compared to those in the control group in KN024 and KN010. This analysis may have resulted in a conservative estimate of Q-TWiST improvement for pembrolizumab.
The EQ-5D-3L has been noted to have potential limitations (e.g., ceiling effects) as well as issues with its implementation in oncology clinical trials [34]. In KN024 and KN010, EQ-5D-3L was only administered for discontinued patients at the discontinuation visit and 30 days thereafter. The derivation of the utility value for the REL state could be subject to clear uncertainty. We address this via sensitivity analyses to assess the impact of utilities. In line with historical Q-TWiST analyses, the standardized utility values were used, which led to even more favorable Q-TWiST results for pembrolizumab compared with the base-case results. In addition, sensitivity analyses on a panel of utility values demonstrated that the Q-TWiST improvement of pembrolizumab compared to chemotherapy was statistically significant and clinically meaningful regardless of utility values assigned.
Limitations may also be acknowledged in utilization of the traditional Q-TWiST methodology in assessment of novel therapies. First, the so-called pseudo-progression [35] associated with immunotherapies may imply that the traditional definition of progression can be inadequate to evaluate clinical response and consequently an alternative endpoint may be needed to more accurately capture disease progression for immunotherapies. Second, the current Q-TWiST framework does not incorporate AEs that occur or last beyond the time of progression. Considering that patients deriving clinical benefit could continue immunotherapy beyond progression, exploring the impact of incorporating TOX in the REL state would be valuable. Finally, immunotherapies are often associated with delayed treatment effects and long-term survivors [36]. The traditional Q-TWiST framework, which does not take into account patient heterogeneity and long-term survival, may underestimate the clinical benefits of immunotherapies. Therefore, further research may enhance the Q-TWiST method to better assess the benefit–risk of immunotherapies.
5 Conclusions
Pembrolizumab was associated with statistically significant and clinically meaningful improvement in quality-adjusted survival using the Q-TWiST analysis compared to chemotherapy in PD-L1–positive metastatic NSCLC in both previously untreated and treated patients. The benefits continued to accrue over the trial follow-up period. Relative Q-TWiST gains for pembrolizumab compare favorably with the reported standardized results for immune-oncology therapies. The Q-TWiST approach provides information on the benefit–risk associated with treatments in cancer that complements other approaches used to evaluate and compare health outcomes.
References
Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomark Prev. 2016;25(1):16–27.
Ettinger DS, et al. Non–small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–35.
Cho JH. Immunotherapy for non-small-cell lung cancer: current status and future obstacles. Immune Netw. 2017;17(6):378–91.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Investigators K-. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Brahmer J, et al. OA 17.06 updated analysis of KEYNOTE-024: Pembrolizumab vs platinum-based chemotherapy for advanced NSCLC with PD-L1 TPS ≥ 50%. J Thorac Oncol. 2017;12(11):S1793–4.
Lopes G, Wu Y, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(18_suppl):LBA4.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE 010): a randomised controlled trial. Lancet. 2016;387:1540–50.
Herbst RS, et al. Factors associated with better overall survival (OS) in patients with previously treated, PD-L1–expressing, advanced NSCLC: Multivariate analysis of KEYNOTE-010. J Clin Oncol. 2017;35(15_suppl):9090. https://doi.org/10.1200/JCO.2017.35.15_suppl.9090.
Mt-Isa S, Hallgreen CE, Wang N, et al. on behalf of the IMI-PROTECT benefit–risk participants. Balancing benefit and risk of medicines: a systematic review and classification of available methodologies. Pharmacoepidemiol Drug Saf. 2014;23(7):667–78.
Guo JJ, Swapnil P, Doyle J, Boyang B, Lis Y, Raisch DW. A review of quantitative risk-benefit methodologies for assessing drug safety and efficacy—report of the ISPOR risk-benefit management working group. Value Health. 2010;13:657–66.
Huang M, Lou Y, Pellissier J, Burke T, Liu FX, Xu R, Velcheti V. Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Pharmacoeconomics. 2017;35(8):831–44.
Huang M, Lou Y, Pellissier J, Burke T, Liu FX, Xu R, Velcheti V. Cost-effectiveness of pembrolizumab versus docetaxel for the treatment of previously treated PD-L1 positive advanced NSCLC patients in the United States. J Med Econ. 2017;20(2):140–50.
Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology Value Framework: revisions and reflections in response to comments received. J Clin Oncol. 2016;34(24):2925–34.
European Medicines Agency. Revised framework for interaction between the European Medicines Agency and patients and consumers and their organisations; 2014. http://attwww.ema.europa.eu/docs/en_GB/document_library/Other2009/12/WC500018013.pdf. Accessed 18 Nov 2018.
US. Food and Drug Administration. Plan for issuance of patient-focused drug development guidance under 21st century cures act title III section 3002; 2017. https://www.fda.gov/downloads/forindustry/userfees/prescriptiondruguserfee/ucm563618.pdf. Accessed 18 Nov 2018.
Gelber RD, Goldhirsch A. A new endpoint for the assessment of adjuvant therapy in postmenopausal women with operable breast cancer. J Clin Oncol. 1986;4:1772–9.
Goldhirsch A, Gelber RD, Simes RJ, Glasziou P, Coates AS. Costs and benefits of adjuvant therapy in breast cancer: a quality-adjusted survival analysis. J Clin Oncol. 1989;7(1):36–44.
Gelber RD, Goldhirsch A, Cavalli F. Quality-of-life-adjusted evaluation of adjuvant therapies for operable breast cancer. The International Breast Cancer Study Group. Ann Intern Med. 1991;114:621–8.
Zbrozek AS, Hudes G, Levy D, Strahs A, Berkenblit A, De Marinis R, Parasuraman S. Q-TWiST analysis of patients receiving temsirolimus or interferon alpha for treatment of advanced renal cell carcinoma. Pharmacoeconomics. 2010;28(7):577–84.
Sherrill B, Wang J, Kotapati S, Chin K. Q-twist analysis comparing ipilimumab/dacarbazine vs. placebo/dacarbazine for patients with stage iii/iv melanoma. Br J Cancer. 2013;109(1):8–13.
Patil S, Figlin RA, Hutson TE, Michaelson MD, Negrier S, Kim ST, Huang X, Motzer RJ. Q-twist analysis to estimate overall benefit for patients with metastatic renal cell carcinoma treated in a phase iii trial of sunitinib vs interferon-α. Br J Cancer. 2012;106(10):1587–90.
McDermott DF, Shah R, Gupte-Singh K, Sabater J, Luo L, Botteman M, Rao S, Regan MM, Atkins M. Quality-adjusted survival of nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone among treatment-naive patients with advanced melanoma: a quality-adjusted time without symptoms or toxicity (Q-TWiST) analysis. Qual Life Res. 2018. https://doi.org/10.1007/s11136-018-1984-3.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Common Terminology Criteria for Adverse Events V4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed 24 July 2018.
Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–20.
Dolan P. Modeling valuations for EuroQol health states. Med-Care. 1997;35:1095–108.
Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six country study. Eur J Health Econ. 2003;4:222–31.
Glasziou PP, Simes RJ, Gelber RD. Quality adjusted survival analysis. Stat Med. 1990;9(11):1259–76.
Revicki DA, Feeny D, Hunt TL, Cole BF. Analyzing oncology clinical trial data using the Q-TWiST method: clinical importance and sources for health state preference data. Qual Life Res. 2006;15(3):411–23.
Solem CT, Kwon Y, Shah RM, Aly A, Botteman MF. Systematic review and benchmarking of quality-adjusted time without symptoms or toxicity (Q-TWiST) in oncology. Expert Rev Pharmacoecon Outcomes Res. 2018;18(3):245–53.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Vandormael K, Malbecq W, Pietanza MC, Brahmer J. 188P Treatment switching–adjusted overall survival (OS) in KEYNOTE-024: first-line pembrolizumab versus chemotherapy in patients with advanced non-small cell lung cancer (NSCLC). J Thorac Oncol. 2018;13(4):S112–3.
Huang M, Pellissier J, Kong F. A trial-based Euroqol Eq-5d health utility analysis in patients with previously untreated metastatic NSCLC. In: ISPOR 22nd annual international meeting; Boston, MA 2017 May 20.
Huang M, Pellissier J, Liao J. A trial-based Euroqol EQ-5D health utility analysis in patients with previously treated advanced NSCLC. Value Health. 2016;19(7):A744.
EuroQoL EQ-5D. https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/. Accessed 20 Nov 2018.
Chiou Victoria L, Burotto Mauricio. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33(31):3541.
Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, Soria JC. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol. 2014;9(2):144–53.
Acknowledgements
The authors would like to thank Jane Liao (Merck & Co. Inc.) for her dedicated work on programming support.
Data Availability Statements
Several datasets were used for this analysis: efficacy, safety and patient reported outcomes data collected in phase 3 KEYNOTE-024 and KEYNOTE-010 trials. The patient-level data are not publicly available, but the results of the trials have been presented in several publications. The trial results supporting the findings of this analysis are available within the article. The analysis was conducted in SAS 9.4 and is not publicly available. It is available from the authors upon request, with permission of Merck and Co. Inc. and a signed confidentiality agreement.
Author information
Authors and Affiliations
Contributions
MH led the development of this analysis and had full access to all the data in the study. MH takes responsibility for the integrity of the data and the accuracy of the analysis. All authors contributed to the study design and methodology, interpretation of the results, development and writing of the draft manuscript, and approved the final version.
Corresponding author
Ethics declarations
Funding
This study and manuscript were funded by Merck & Co. Inc. MH, MCP, AS, JP, TB, SC and FK are employees of Merck & Co. Inc., the sponsor of this study and manuscript. ASP provides advisory and consultancy services to the EuroQol Group, Merck, Bristol-Myers Squibb, and Novartis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Huang, M., Pietanza, M.C., Samkari, A. et al. Q-TWiST Analysis to Assess Benefit–Risk of Pembrolizumab in Patients with PD-L1–Positive Advanced or Metastatic Non-small Cell Lung Cancer. PharmacoEconomics 37, 105–116 (2019). https://doi.org/10.1007/s40273-018-0752-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0752-0