Abstract
Background
Adult-onset myotonic dystrophy type 1 (DM1) is a chronic, multisystem disorder that leads to disability and premature death.
Objectives
The objective of our study was to conduct a systematic literature review of the health-related quality of life (HRQoL) of patients with DM1.
Methods
We searched Embase, Web of Science, and PubMed for English language full-text articles reporting results from studies of HRQoL in patients with adult-onset DM1 published between 1 January 2000 and 21 February 2018. We excluded reviews, editorials, and studies reporting results for a sample with fewer than five patients (to allow for meaningful inference).
Results
The search identified 266 unique publications. Of these, 231 were excluded following title and abstract screening and 16 after full-text review, leaving 19 articles for data synthesis. We found 15 articles measuring the HRQoL of patients with adult-onset DM1 using the 36-Item Short Form Health Survey (SF-36), six using the Individualized Neuromuscular Quality of Life Questionnaire (INQoL), and one using Cantril’s Ladder. Available evidence shows that patient HRQoL is impaired in DM1, mainly due to compromised physical health, but also reveals that substantial heterogeneity exists in estimates across studies.
Conclusions
HRQoL in adult-onset DM1 has been extensively studied using the SF-36 and the INQoL, but current estimates are inconclusive, and little is known of the impact of the disease as measured using other instruments. Our data synthesis should help characterize the patient burden of DM1 and inform future studies of HRQoL in this indication.
Similar content being viewed by others
A range of studies has examined health-related quality of life (HRQoL) in myotonic dystrophy type 1 (DM1) using the 36-Item Short Form Health Survey and the Individualized Neuromuscular Quality of Life Questionnaire, but exploration using other instruments is lacking (particularly preference-based scales). |
Our review of HRQoL in DM1 reveals substantial heterogeneity in published estimates of the latent trait across studies and samples. |
Our synthesis contributes to the understanding of the health burden of the disease and should help to inform future research of HRQoL in DM1. |
1 Introduction
Myotonic dystrophy type 1 (DM1) is a chronic, progressive multisystem disorder of variable severity that leads to disability and premature death [1]. It is inherited in an autosomal dominant manner and represents the most common muscular dystrophy in adults, with an estimated prevalence in Europe of between 10 and 18 per 100,000 people [2]. For many patients, DM1 is associated with an array of different complications, including but not limited to muscle weakness, fatigue, cardiac conduction defects, excessive daytime sleepiness (EDS), endocrine disturbance, and gastrointestinal problems [1, 3]. It is also common for several members of the same family to be affected, resulting in a substantial health burden at the household level. Currently, only symptomatic treatments are available, but potentially more effective therapies are under investigation [3].
A number of recent studies have investigated the health-related quality of life (HRQoL) of patients with disabling neuromuscular diseases, including DM1. The aim of our study was to review the literature about the HRQoL of patients with adult-onset DM1. Specifically, this systematic literature review sought to answer the following questions:
-
In which geographical settings has the HRQoL of patients with adult-onset DM1 been studied?
-
What instruments have been used to measure the HRQoL of patients with adult-onset DM1?
-
What is known of the HRQoL of patients with adult-onset DM1?
2 Methods
This systematic literature review was conducted and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [4].
2.1 Search Strategy
We searched Embase, Web of Science, and PubMed for full-text articles reporting results from studies of HRQoL in DM1. The search string contained a combination of the following medical subject heading terms, title/abstract, and topic field tags: myotonic dystrophy, myotonic dystrophy type 1, DM1, Steinert’s disease, quality of life, health-related quality of life, utility, and well-being (see the Electronic Supplementary Material [ESM] for the full search strings). We excluded (1) articles published before the year 2000 (to ensure estimates of HRQoL reflected current standard-of-care practices), (2) reviews and editorials, (3) articles in a language other than English, and (4) studies reporting results for a sample of fewer than five patients (to allow for meaningful inference). For studies including patients with different indications, we also required that results were reported separately for patients with adult-onset DM1. Given our objective was to review studies of HRQoL, we did not include publications only reporting data concerning specific disease complications, manifestations, or domains of psychological or physical health, for example, depression, pain, or fatigue, and we also excluded congenital myotonic dystrophy and childhood/juvenile-onset myotonic dystrophy (as the measurement of HRQoL in pediatric populations requires specific considerations [5]). Lastly, we excluded studies only reporting changes in measures of HRQoL or correlations between HRQoL and other outcomes/instruments.
2.2 Screening, Data Extraction, and Synthesis of Results
The search was performed on 21 February 2018. Two independent investigators (EL and JE) initially screened article titles and abstracts for eligibility and subsequently reviewed the full-text versions of selected records. The following data were extracted from all articles included in the review: author, year of publication, setting, sample, methods for measuring HRQoL (including employed instruments), and main results. The reasons for article exclusion were recorded, and potential disagreements were resolved by consensus or, if necessary, with the involvement of a third investigator (HL). Results data from each article were synthesized and reported with respect to the three review questions listed in Sect. 1. Data presented in graphs were extracted using a specialized graph-digitizing software (DigitizeIt). We did not compare results from generic instruments with general population reference data (where available), as published normative estimates have been shown to vary across, for example, geographical settings and age groups and by sex [6]. We assessed study quality and risk of bias using the National Institute of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [7].
3 Results
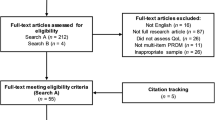
The systematic literature review identified 469 publications (Fig. 1). Of these, 203 were duplicates, 231 records were excluded following title and abstract screening, and 35 articles were selected for full-text review. Finally, 19 articles [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] were considered for data synthesis. Table 1 summarizes the data from the included publications and the outcomes from the study quality and bias assessments. Only one study [17] was judged to be of poor quality because of unclear reporting and validity of the outcome of interest.
3.1 In Which Geographical Settings has the Health-Related Quality of Life (HRQoL) of Patients with Adult-Onset Myotonic Dystrophy Type 1 (DM1) been Studied?
We identified estimates of HRQoL for patients with adult-onset DM1 from eight countries from three continents (Europe, North America, and South America) (Table 1). With the exception of Serbia, which accounted for 37% (7 of 19) of all identified records, studies of samples from other countries were limited to one, two, or three publications.
3.2 What Instruments Have Been Used to Measure the HRQoL of Patients with Adult-Onset DM1?
In total, 15 articles [8, 9, 11,12,13,14, 17,18,19,20,21,22,23,24, 26] used the 36-Item Short Form Health Survey (SF-36), six [9, 10, 15, 16, 23, 25] used the Individualized Neuromuscular Quality of Life Questionnaire (INQoL), and one [24] used Cantril’s Ladder (CL) to measure the HRQoL of patients with adult-onset DM1.
3.3 What is Known of the HRQoL of Patients with Adult-Onset DM1?
Figures 2 and 3 summarize the results from studies using the SF-36 instrument (comprising eight subscales, as well as two summary measures, each scored between 0 and 100, where a higher score represents greater HRQoL). Three studies [8, 19, 26] found that patients had lower scores across all SF-36 subscales than matched controls from the general population or general population reference data, whereas two [20, 24] reported lower physical health but not mental health (MH). Across studies and sample populations, mean scores were as follows: physical functioning (PF; 38–82), role physical (RP; 22–95), bodily pain (BP; 51–82), general health (GH; 34–77), vitality (31–69), social functioning (SF; 56–93), role emotional (RE; 28–96), and MH (53–81). Mean physical component summary (PCS) and mental component summary (MCS) scores, respectively, were estimated at 38 and 41 [13], 42 and 53 [20], 42 and 47 [12], 52 and 56 [14], 70 and 76 [14], 37 and 48 [24], 42 and 45 (baseline visit) [11], and 50 and 59 (follow-up visit) [11]. In addition, one study published a mix of median and mean SF-36 scores. Specifically, Sansone et al. [9] estimated the median scores as PF 60, RP 75, BP 82, SF 75, RE 100, MH 68, PCS 49, and MCS 51 and mean scores as GH 45 and vitality 51. Only one study [23] reported HRQoL in terms of utilities derived from the SF-36 (through the Short Form Six-Dimensions [SF-6D] algorithm). The median utility was estimated at 0.710 for employed patients and 0.678 for unemployed patients (utility estimates for other subgroups, or the pooled sample, were not reported). It should be noted that one study [17] appeared to report SF-36 scores incorrectly (the median “SF-36/quality of life” was estimated at >100).
Health-related quality of life of patients with adult-onset myotonic dystrophy type 1 as measured using the. A higher score represents greater health-related quality of life. For references, see Table 1. We only included baseline results from the study by O’Donoghue et al. [18] because there were no significant differences across follow-up. Subgroups from Laberge et al. [21]: [A] fatigue, [B] fatigue and excessive daytime sleepiness, [C] excessive daytime sleepiness, and [D] neither fatigue nor excessive daytime sleepiness. Subgroups from Peric et al. [11]: [E] baseline and [F] after 5 years
Health-related quality of life of patients with adult-onset myotonic dystrophy type 1 as measured using the 36-Item Short Form Health Survey (SF-36). A higher score represents greater health-related quality of life. For references, see Table 1. We only included baseline results from the study by O’Donoghue et al. [18] because there were no significant differences across follow-up. No estimate for vitality was published by Kalkman et al. [22]. Subgroups from Laberge et al. [21]: [A] fatigue, [B] fatigue and excessive daytime sleepiness, [C] excessive daytime sleepiness, and [D] neither fatigue nor excessive daytime sleepiness. Subgroups from Peric et al. [11]: [E] baseline and [F] after 5 years
Figure 4 presents the mean INQoL results, where a higher score represents a higher disease burden and lower HRQoL. Across studies and sample populations, mean scores ranged as follows: muscle weakness (49–70), muscle locking (38–63), pain (25–42), fatigue (42–60), activities (35–55), independence (30–47), social relationships (10–23), emotions (24–44), and body image (6–62). In addition, one study published median INQoL scores. Specifically, Sansone et al. [9] estimated median scores across instrument domains as 47, 32, 0, 42, 29, 17, 4, 19, and 25, respectively. Heatwole et al. [23] reported only total INQoL scores for patients who were employed (29) and unemployed (42).
Health-related quality of life of patients with adult-onset myotonic dystrophy type 1 as measured using the Individualized Neuromuscular Quality of Life Questionnaire (INQoL). A higher score represents lower health-related quality of life. For references, see Table 1. No estimate for pain was published by Peric et al. [16]. Subgroups from Peric et al. [15]: [A] baseline and [B] after 5 years
Lastly, Geirdal et al. [24] estimated the mean score from CL (single-item scale ranging from 0 = worst possible quality of life to 10 = best possible quality of life) as 5.85. This estimate was not statistically different from the mean score of healthy relatives or next of kin without DM1.
4 Discussion
The aim of this study was to conduct a systematic literature review of studies investigating the HRQoL of patients with adult-onset DM1. Taken together, our results show that HRQoL in this patient population is well-explored using the SF-36 and the INQoL in several settings, but estimates from other rating scales are lacking, particularly preference-based instruments (i.e., instruments linked to utilities).
As shown in Figs. 2 and 3, our synthesis of patient HRQoL as measured through the SF-36 revealed a trend of impairment (in relation to the maximum score of 100) across all instrument subscales, particularly RP, GH, and vitality, yet estimates varied substantially between studies. This heterogeneity may be related to differences in patient samples (e.g., disease severity and prevalence of complications, as well as demographical and cultural factors) [6] but may also be a function of the psychometric properties of the scale. For instance, the SF-36 is an ordinal scale and would thus not be expected to fulfil the strict criteria of fundamental measurement, which is a requirement for invariant comparison [27, 28]. Indeed, although it is clear that a lower/higher score indicates lower/higher HRQoL, how a specific score, or differences in scores at various points across the continuum, should be interpreted is unknown. The notable variability in point estimates from the SF-36 identified in this review also indicates that the rating scale may not achieve invariance, which means it is affected by the sample it is measuring (i.e., not “stable”) [28]. This means that the estimated continuum, and the location of items on the continuum, is not sample independent and that the scale fails to “transcend the group measured” [29]. For these reasons, it may not be meaningful to directly compare mean estimates across study samples, or even between patients, or with general population reference data. Accordingly, the validity of the SF-36 as a measure of HRQoL in patients with adult-onset DM1 warrants further investigation, preferably using modern psychometric methods, such as Rasch analysis [30]. Thus, in agreement with Peric et al. [11], we suggest that estimates from the SF-36 in this indication should be interpreted with caution.
Similar to outcomes from the SF-36, our synthesis of results from the INQoL indicated impairment in patient HRQoL across many instrument domains, particularly muscle weakness, muscle locking, and fatigue, but also revealed non-trivial differences between assessments (Fig. 4). Given its scoring structure, the INQoL would be expected to be subject to similar psychometric issues as noted for the SF-36. That being said, the instrument records a relatively rich set of information on a range of aspects that would be expected to be associated with HRQoL—including self-perceived disease burden; amount and perceived importance of symptoms; ability to perform daily, work, and leisure activities; and impact on relationships—so we think it may still, depending on study design, be of interest and relevance to include the tool in future research involving patients with DM1.
The lack of psychometric data for scales used to measure latent traits, such as health status, HRQoL, and ability to perform activities of daily living, in patients with DM1 was also noted in a recently published literature review of patient-reported outcome measures in this indication [31]. In particular, the authors found that the validity of many instruments has not been thoroughly investigated and that disease-specific properties, such as responsiveness and minimal clinically important difference thresholds, are needed for all measures (although it should be noted that the SF-36 was not included in the review). Further research into these topics is strongly encouraged to ensure the tools employed are fit for purpose [32].
We only identified two point estimates of patient HRQoL in terms of utilities. This was a bit surprising given that outcomes from the SF-36 can be mapped to utilities (through the SF-6D). In general, utility estimates derived from members of the general population would be expected to provide additional, complementary information about the patient burden of a disease, as they reflect preferences for health states (as dictated by the disease, among other factors) from the perspective of healthy individuals ex ante experiencing the health state. In fact, there are several reasons why patients and members of the general population would attribute a different preference weight to a specific health state (e.g., being affected by DM1). First, individuals from the general population may be subject to a process known as “focalism” or “focusing illusion” [33, 34], where positive aspects of a health state are underestimated or neglected because the attention is focused on negative consequences. Second, patients may learn to cope with their illness and/or disability, adjust their perception and expectations of HRQoL, and adapt to their health state, a phenomenon known as “response shift” or “well-being paradox” [5]. Evidence of such coping mechanisms has been noted in previous research in patients with DM1 [11, 15] or other genetic, chronic illnesses, such as Duchenne muscular dystrophy [35] or cerebral palsy [36]. Third, patients and the general population may have different vantage points, with patients assessing their health state in terms of the benefits that would result from regaining health, whereas members of the general population may view the health state in terms of the costs associated with losing good health [37]. As a consequence, among patients, changes in health states governing HRQoL may not be fully detected using rating scales such as the SF-36 and INQoL. On the other hand, estimates of HRQoL measured in terms of utilities will vary across health states as classified by the instruments. Yet, it is not certain that instruments linked to utilities map out health states (using the included items and levels) that are relevant to DM1, or the efficacy of interventions in the context of clinical trials, and may therefore lack sensitivity. This would be expected to be the case in DM1 with, for example, a very short, generic instrument such as the EuroQol Five-Dimensions (EQ-5D). Moreover, bearing in mind that appraisals of pricing and reimbursement of orphan drugs in most countries also include evidence of cost effectiveness [38], estimates of HRQoL in terms of utilities will also be important to facilitate economic evaluations of future health technologies targeting DM1.
Although of uncertain relevance given the psychometric limitations of the SF-36 and INQoL as noted, several studies have explored how these tools correlate with other measures in patients with DM1. Total and subscale scores from the SF-36 have been shown to be inversely correlated with, for example, age [11,12,13, 22,23,24,25,26]; duration and/or severity of disease [11,12,13, 20, 21, 26]; fatigue [12, 20, 22]; depression, anxiety, psychological distress, and/or neuroticism [11,12,13, 20, 21, 24, 26]; intellectual quotient [20]; and EDS [20] and positively correlated with education [11,12,13]. Moreover, the INQoL has been shown to be positively correlated with, for example, age [10], measures of disease severity [10, 23], depression and anxiety [10], and pain [16] and inversely correlated with education [10]. However, it is worth noting that these findings are not exhaustive, and studies may have reached alternative conclusions with regards to the correlations between SF-36 and INQoL total and subscale scores, respectively, and other variables.
The main clinical implication of this review, in addition to informing the design of and selection of endpoints for future patient trials of new interventions (as discussed), concerns the identified variability in estimates within the current body of evidence, indicative of the heterogeneous presentation of DM1. Indeed, evident from our data synthesis, DM1 appears to have a highly variable but nontrivial impact (in relation to maximum scale scores) across many domains of patients’ lives, underscoring the need for a patient-centered and holistic approach to disease management. For this reason, it may also be of interest to further explore the burden of DM1 on informal caregivers (e.g., partners, family members, and close friends), who often play an integral part in the provision of long-term care in many illness [39].
5 Conclusion
HRQoL in adult-onset DM1 has been extensively studied using the SF-36 and the INQoL, but current estimates are inconclusive and little is known of the impact of the disease as measured using other instruments. Our data synthesis should help characterize the patient burden of DM1 and inform future studies of HRQoL in this indication.
References
Wood L, Bassez G, van Engelen B, et al. 222nd ENMC International Workshop: myotonic dystrophy, developing a European consortium for care and therapy, Naarden, The Netherlands, 1–2 July 2016. Neuromuscul Disord. 2018;28(5):463–9.
Lindberg C, Bjerkne F. Prevalence of myotonic dystrophy type 1 in adults in western Sweden. Neuromuscul Disord. 2017;27(2):159–62.
Smith CA, Gutmann L. Myotonic dystrophy type 1 management and therapeutics. Curr Treat Options Neurol. 2016;18(12):52.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Eiser C, Morse R. Quality-of-life measures in chronic diseases of childhood. Health Technol Assess. 2001;5(4):1–157.
Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey. Manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993.
National Institute of Health. Study Quality Assessment Tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 10 Sep 2018.
Tieleman AA, Jenks KM, Kalkman JS, Borm G, Van Engelen BGM. High disease impact of myotonic dystrophy type 2 on physical and mental functioning. J Neurol. 2011;258(10):1820–6.
Sansone VA, Ricci C, Montanari M, et al. Measuring quality of life impairment in skeletal muscle channelopathies. Eur J Neurol. 2012;19(11):1470–6.
Rakocevic-Stojanovic V, Peric S, Madzarevic R, et al. Significant impact of behavioral and cognitive impairment on quality of life in patients with myotonic dystrophy type 1. Clin Neurol Neurosurg. 2014;126:76–81.
Peric S, Vujnic M, Dobricic V, et al. Five-year study of quality of life in myotonic dystrophy. Acta Neurol Scand. 2016;134(5):346–51.
Peric S, Stojanovic VR, Basta I, et al. Influence of multisystemic affection on health-related quality of life in patients with myotonic dystrophy type 1. Clin Neurol Neurosurg. 2013;115(3):270–5.
Peric S, Rakocevic-Stojanovic V, Stevic Z, et al. Health-related quality of life in patients with myotonic dystrophy type 1 and amyotrophic lateral sclerosis. Acta Neurol Belg. 2010;110(1):71–7.
Peric S, Nisic T, Milicev M, et al. Hypogonadism and erectile dysfunction in myotonic dystrophy type 1. Acta Myol. 2013;32(2):106–9.
Peric S, Heatwole C, Durovic E, et al. Prospective measurement of quality of life in myotonic dystrophy type 1. Acta Neurol Scand. 2017;136(6):694–7.
Peric M, Peric S, Rapajic N, et al. Multidimensional aspects of pain in myotonic dystrophies. Acta Myol. 2015;34(2–3):126–32.
Orlikowski D, Chevret S, Quera-Salva MA, et al. Modafinil for the treatment of hypersomnia associated with myotonic muscular dystrophy in adults: a multicenter, prospective, randomized, double-blind, placebo-controlled, 4-week trial. Clin Ther. 2009;31(8):1765–73.
O’Donoghue FJ, Borel JC, Dauvilliers Y, Levy P, Tamisier R, Pépin JL. Effects of 1-month withdrawal of ventilatory support in hypercapnic myotonic dystrophy type 1. Respirology. 2017;22(7):1416–22.
Araújo TL, Resqueti VR, Bruno S, et al. Respiratory muscle strength and quality of life in myotonic dystrophy patients. Rev Port Pneumol. 2010;16(6):892–8.
Laberge L, Mathieu J, Auclair J, Gagnon E, Noreau L, Gagnon C. Clinical, psychosocial, and central correlates of quality of life in myotonic dystrophy type 1 patients. Eur Neurol. 2013;70(5–6):308–15.
Laberge L, Dauvilliers Y, Begin P, Richer L, Jean S, Mathieu J. Fatigue and daytime sleepiness in patients with myotonic dystrophy type 1: to lump or split? Neuromuscul Disord. 2009;19(6):397–402.
Kalkman JS, Schillings ML, van der Werf SP, et al. Experienced fatigue in facioscapulohumeral dystrophy, myotonic dystrophy, and HMSN-I. J Neurol Neurosurg Psychiatry. 2005;76(10):1406–9.
Heatwole C, Johnson N, Dekdebrun J, et al. Myotonic dystrophy patient preferences in patient-reported outcome measures. Muscle Nerve 2018.
Geirdal AØ, Lund-Petersen I, Heiberg A. Understanding the experience of myotonic dystrophy. Mixed method study. J Genet Couns. 2015;24(1):169–78.
Baldanzi S, Bevilacqua F, Lorio R, et al. Disease awareness in myotonic dystrophy type 1: an observational cross-sectional study. Orphanet J Rare Dis. 2016;11(1):34.
Antonini G, Soscia F, Giubilei F, et al. Health-related quality of life in myotonic dystrophy type 1 and its relationship with cognitive and emotional functioning. J Rehabil Med. 2006;38(3):181–5.
Wright B. A history of social science and measurement. Educ Meas. 1997;52:33–52.
Hobart J, Cano S. Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods. Health Technol Assess. 2009;13(12):1–177 (iii, ix–x).
Thurstone LL. Attitudes can be measured. Am J Sociol. 1928;23:529–54.
Rasch G. Probabilistic models for some intelligence and attainment tests (1st Edition). Copenhagen: Danish Institute for Education Research; 1960.
Symonds T, Randall JA, Campbell P. Review of patient-reported outcome measures for use in myotonic dystrophy type 1 patients. Muscle Nerve. 2017;56(1):86–92.
Hobart JC, Cano SJ, Zajicek JP, et al. Rating scales as outcome measures for clinical trials in neurology: problems, solutions, and recommendations. Lancet Neurol. 2007;6:1094–105.
Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6):599–607.
Wilson TD, Wheatley T, Meyers JM, Gilbert DT, Axsom D. Focalism: a source of durability bias in affective forecasting. J Pers Soc Psychol. 2000;78(5):821–36.
Landfeldt E, Lindgren P, Bell CF, Guglieri M, Straub V, Lochmuller H, et al. Health-related quality of life in patients with Duchenne muscular dystrophy: a multinational, cross-sectional study. Dev Med Child Neurol. 2016;58(5):508–15.
Colver A, Rapp M, Eisemann N, et al. Self-reported quality of life of adolescents with cerebral palsy: a cross-sectional and longitudinal analysis. Lancet. 2015;385(9969):705–16.
Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6):599–607.
Gammie T, Lu CY, Babar ZU. Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. PLoS One. 2015;10(10):e0140002.
Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311:1052–60.
Author information
Authors and Affiliations
Contributions
Drs Landfeldt and Lochmüller conceptualized and designed the study, Dr. Landfeldt and Ms. Edström conducted the literature review, Dr. Landfeldt led the interpretation of findings and drafted the manuscript, and Dr. Landfeldt and Ms. Edström critically reviewed the manuscript for important intellectual content. Dr. Jimenez-Moreno, Dr. van Engelen, Dr. Kirschner, and Dr. Lochmüller interpreted the findings and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflicts of Interest
Dr. Landfeldt, Ms. Edström, Dr. Jimenez-Moreno, Dr. van Engelen, Dr. Kirschner, and Dr. Lochmüller have no conflicts of interest that are directly relevant to the content of this article.
Data Availability
All data generated or analysed during this study are included in the published article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Landfeldt, E., Edström, J., Jimenez-Moreno, C. et al. Health-Related Quality of Life in Patients with Adult-Onset Myotonic Dystrophy Type 1: A Systematic Review. Patient 12, 365–373 (2019). https://doi.org/10.1007/s40271-019-00357-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-019-00357-y