Abstract
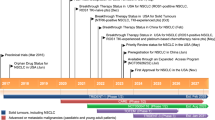
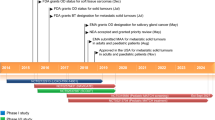
Entrectinib (Rozlytrek®) is an oral selective inhibitor of the tyrosine kinases tropomyosin receptor kinases (Trk)A/B/C [encoded by the genes neurotrophic tyrosine receptor kinase (NTRK) 1, 2 and 3, respectively], c-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK) with central nervous system (CNS) activity developed by Roche for the treatment of various solid tumours harbouring NTRK1/2/3 or ROS1 gene fusions. In June 2019, entrectinib received its first global approval in Japan, for the treatment of adult and paediatric patients with NTRK fusion-positive, advanced or recurrent solid tumours and is under regulatory review for the treatment of adult patients with ROS1-positive non-small cell lung cancer (NSCLC). Entrectinib is also under regulatory review in the USA (PDUFA date 18 August 2019) and EU [Priority Medicines (PRIME) designation] for NTRK-positive solid tumours and ROS1-positive NSCLC. This article summarizes the milestones in the development of entrectinib leading to this first global approval for solid tumours in Japan.
Similar content being viewed by others
References
Liu D, Offin M, Harnicar S, et al. Entrectinib: an orally available, selective tyrosine kinase inhibitor for the treatment of NTRK, ROS1, and ALK fusion-positive solid tumors. Ther Clin Risk Manag. 2018;14:1247–52.
Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–47.
Rangaraju S, Farago A, Heym KM, et al. Preclinical and clinical efficacy of entrectinib in primary and metastatic brain tumors harboring NTRK, ROS1, or ALK gene fusions [abstract no. P14.19]. Neuro Oncol. 2017;19(Suppl. 3):iii106.
Ardini E, Menichincheri M, Banfi P, et al. Entrectinib, a pan-TRK, ROS1, and ALK inhibitor with activity in multiple molecularly defined cancer indications. Mol Cancer Ther. 2016;15(4):628–39.
Menichincheri M, Ardini E, Magnaghi P, et al. Discovery of entrectinib: a new 3-aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ros oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (Pan-TRKs) inhibitor. J Med Chem. 2016;59(7):3392–408.
Roche. Japan becomes the first country to approve Roche’s personalised medicine Rozlytrek [media release]. 18 Jun 2019. https://www.roche.com/media/releases/med-cor-2019-06-18.htm.
Chugai Pharmaceutical Co. Ltd. Rozlytrek (entrectinib) capsules: Japanese prescribing information. 2019. http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/450045_42910D6M1020_1_01. Accessed 8 July 2019.
Ignyta Inc. Ignyta completes merger and announces license agreement for the development of two leading tyrosine kinase inhibitors [media release]. 1 Nov 2013. http://www.ignyta.com.
Chugai Pharmaceutical Co. Ltd. Chugai to in-license ROS1/TRK inhibitor entrectinib [media release]. 17 Jul 2018. http://www.chugai-pharm.co.jp.
Doebele R, Ahn M, Siena S, et al. Efficacy and safety of entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small cell lung cancer (NSCLC) [abstract no. OA02.01 and presentation]. J Thorac Oncol. 2018;13(10 Suppl.):S321–S322.
Anderson D, Ciomei M, Banfi P, et al. Inhibition of Trk-driven tumors by the pan-Trk inhibitor RXDX-101 [abstract no. 310]. Eur J Cancer. 2014;50(Suppl.6):101.
Desai AV, Brodeur GM, Foster J, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors [abstract no. 10536]. J Clin Oncol. 2018;36(15 Suppl.).
Iyer R, Wehrmann L, Golden RL, et al. Entrectinib is a potent inhibitor of Trk-driven neuroblastomas in a xenograft mouse model. Cancer Lett. 2016;372(2):179–86.
Wei G, Ardini E, Patel R, et al. Entrectinib is effective against the gatekeeper and other emerging resistance mutations in NTRK-, ROS1-and ALK-rearranged cancers [abstract no. 2136]. Cancer Res. 2016;76(14 Suppl.).
Wei G, Patel R, Walsh C, et al. Entrectinib, a highly potent pan-Trk, ROS1, and ALK inhibitor, has broad-spectrum, histology-agnostic anti-tumor activity in molecularly defined cancers [abstract no. 78]. Eur J Cancer. 2016;69(Suppl. 1):S33.
Fagan P, Barrera M, Walsh C, et al. Anti-tumor activity of entrectinib, a highly potent pan-TRK, ROS1 and ALK inhibitor, in molecularly defined acute myeloid leukemia [abstract no. 5158]. Cancer Res. 2017;77(13 Suppl. 1).
Smith KM, Fagan PC, Pomari E, et al. Antitumor activity of entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor, in ETV6-NTRK3-positive acute myeloid leukemia. Mol Cancer Ther. 2018;17(2):455–63.
Couts KL, Murphy D, Christiansen J, et al. ROS1 and ALK fusions are novel therapeutic targets in non-sun exposed subtypes of melanoma [abstract no. CS.16.03]. Pigment Cell Melanoma Res. 2017;30(5):e61.
Patel MR, Bauer TM, Liu SV, et al. STARTRK1: Phase 1/2a study of entrectinib, an oral Pan-Trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations [abstract no. 2596 and poster]. J Clin Oncol. 2015;33(15 Suppl. 1).
Demetri GD, Paz-Ares L, Farago AF, et al. Efficacy and safety of entrectinib in patients with NTRK fusion-positive tumours: pooled analysis of STARTRK- 2, STARTRK-1, and ALKA-372-001 [abstract no. LBA4]. Ann Oncol. 2018;29(Suppl. 9):ix175.
Siena S, Doebele RC, Shaw AT, et al. Efficacy of entrectinib in patients (pts) with solid tumors and central nervous system (CNS) metastases: integrated analysis from three clinical trials [abstract no. 3017 plus oral presentation]. J Clin Oncol. 2019;37(15 Suppl.).
ClinicalTrials.gov. Basket study of entrectinib (RXDX-101) for the treatment of patients with solid tumors harboring NTRK 1/2/3 (Trk A/B/C), ROS1, or ALK gene rearrangements (fusions) (STARTRK-2). 2015. https://clinicaltrials.gov/ct2/show/NCT02568267?term=NCT02568267&rank=1. Accessed 18 July 2019.
Barlesi F, Drilon A, De Braud F, et al. Entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small cell lung cancer (NSCLC): integrated analysis of ALKA-372-001, STARTRK-1 and STARTRK-2 [abstract no. 109O]. Ann Oncol. 2019;30(Suppl. 2):ii42–ii43.
Paz-Ares L, Doebele R, Farago A, et al. Entrectinib in NTRK fusion-positive non-small cell lung cancer (NSCLC): integrated analysis of patients (pts) enrolled in STARTRK-2, STARTRK-1 and ALKA-372-001 [abstract no. 113O]. Ann Oncol. 2019;30(Suppl. 2):ii44–ii45.
Drilon A, Siena S, Ou SI, et al. Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017;7(4):400–9.
Robinson GW, Gajjar AJ, Gauvain KM, et al. Phase 1/1b trial to assess the activity of entrectinib in children and adolescents with recurrent or refractory solid tumors including central nervous system (CNS) tumors [abstract no. 10009]. J Clin Oncol. 2019;37(15 Suppl.).
Desai AV, Brodeur GM, Foster J, et al. STARTRK-NG: A phase 1/1b study of entrectinib in children and adolescents with advanced solid tumors and primary CNS tumors, with or without TRK, ROS1, or ALK fusions [abstract no. CT030]. Cancer Res. 2017;77(13 Suppl. 1).
Abdulla DSY, Doebele RC, Ahn MJ, et al. ENCORE: efficacy and safety of entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small cell lung cancer (NSCLC) [abstract no. P03]. Pneumologie. 2019;73(S01):P623.
ArcherDx. ArcherDX’s companion diagnostic assay for both liquid biopsy and tissue specimens granted breakthrough device designation by U.S. Food and Drug Administration [media release]. 8 Jan 2019. http://www.archerdx.com.
Ignyta Inc. Ignyta announces approval of an investigational device exemption (IDE) for the companion diagnostic assay to the STARTRK-2 trial [media release]. 1 Sep 2016. http://www.ignyta.com.
Chugai Pharmaceutical Co. Ltd. Chugai obtains approval of FoundationOne CDx Cancer Genomic Profile as a companion diagnostic for Rozlytrek [media release]. 27 Jun 2019. https://www.chugai-pharm.co.jp/english/news/detail/20190627120000_628.html.
US FDA. FoundationOne CDx- P170019. 2017. https://www.fda.gov/medical-devices/recently-approved-devices/foundationone-cdx-p170019. Accessed 29 Jul 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Zaina T. Al-Salama and Susan J. Keam are salaried employees of Adis International Ltd/Springer Nature, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
Additional information for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.8980277.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Al-Salama, Z.T., Keam, S.J. Entrectinib: First Global Approval. Drugs 79, 1477–1483 (2019). https://doi.org/10.1007/s40265-019-01177-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01177-y