Abstract
Dolutegravir/rilpivirine (Juluca®) is the first two-drug single-tablet regimen (STR) to be approved for the treatment of HIV-1 infection in adults. The fixed-dose STR combines the integrase strand transfer inhibitor dolutegravir with the non-nucleoside reverse transcriptase inhibitor rilpivirine. In two phase III non-inferiority trials (SWORD-1 and SWORD-2) in treatment-experienced patients already virologically suppressed on their current antiretroviral (ART) regimen, switching to once-daily dolutegravir plus rilpivirine maintained virological suppression over 48 weeks. Switching to a two-drug regimen of dolutegravir plus rilpivirine was also associated with high rates of virological suppression in real-world observational studies. Switching to once-daily dolutegravir plus rilpivirine was generally well tolerated and associated with more favourable renal and bone parameters than remaining on the current ART regimen. Longer-term, dolutegravir plus rilpivirine demonstrated durable maintenance of virological suppression and remained generally well tolerated for up to 100 weeks. Thus, dolutegravir/rilpivirine provides a convenient alternative treatment option for some adults with HIV-1 infection and no history of virological failure who are already virologically suppressed on (and wish to switch from) their current ART regimen.
Similar content being viewed by others
References
Bhatti AB, Usman M, Kandi V. Current scenario of HIV/AIDS, treatment options, and major challenges with compliance to antiretroviral therapy. Cureus. 2016;8(3):e515.
European AIDS Clinical Society. Guidelines version 9.0; 2017. http://eacsociety.org. Accessed 29 Oct 2018.
US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2018. https://adisinfo.nih.gov/guidelines. Accessed 29 Oct 2018.
Boswell R, Foisy MM, Hughes CA. Dolutegravir dual therapy as maintenance treatment in HIV-infected patients: a review. Ann Pharmacother. 2018;52(7):681–9.
de Miguel Buckley R, Montejano R, Stella-Ascariz N, et al. New strategies of ARV: the road to simplification. Curr HIV/AIDS Rep. 2018;15(1):11–9.
Soriano V, Fernandez-Montero JV, Benitez-Gutierrez L, et al. Dual antiretroviral therapy for HIV infection. Expert Opin Drug Saf. 2017;16(8):923–32.
Caplan MR, Daar ES, Corado KC. Next generation fixed dose combination pharmacotherapies for treating HIV. Expert Opin Pharmacother. 2018;19(6):589–96.
European Medicines Agency. Juluca 50 mg/25 mg film-coated tablets: EU summary of product characteristics; 2018. http://www.ema.europa.eu. Accessed 29 Oct 2018.
ViiV Healthcare. JULUCA (dolutegravir and rilpivirine) tablets, for oral use: US prescribing information; 2017. https://www.fda.gov. Accessed 29 Oct 2018.
Kobayashi M, Yoshinaga T, Seki T, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55(2):813–21.
McCormack PL. Dolutegravir: a review of its use in the management of HIV-1 infection in adolescents and adults. Drugs. 2014;74(11):1241–52.
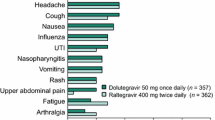
Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839–49.
Mehta R, Wolstenholme A, Di Lullo K, et al. Bioequivalence of a fixed-dose combination tablet of the complete 2-drug regimen dolutegravir and rilpivirine for the treatment of HIV-1 infection. Antimicrob Agents Chemother. 2018;62(9):e00748-18.
Elliot ER, Wang X, Singh S, et al. Increased dolutegravir peak concentrations in people living with HIV aged 60 and over and analysis of sleep quality and cognition. Clin Infect Dis. 2018. https://doi.org/10.1093/cid/ciy426.
Orkin C, Khuong-Josses MA, Lutz T, et al. Safety and efficacy of DTG + RPV in the phase III SWORD-1 and SWORD-2 studies: 48-week subgroup analysis by baseline third agent class and geographic location [abstract no. P22]. HIV Med. 2018;19(Suppl 2):S28–9.
Walmsley S, Richmond GJ, Bredeek F, et al. SWORD-1 and SWORD-2: subgroup analysis of 48-week results by age, race and gender [abstract no. 1382 plus poster]. In: IDWeek; 2017.
Oglesby A, Punekar Y, Angelis K, et al. Patient reported outcomes after switching to a 2-drug regimen of dolutegravir + rilpivirine: results from the SWORD-1 and SWORD-2 studies [abstract no. BPD1/2 plus poster]. In: 16th European AIDS conference; 2017.
Aboud M, Orkin C, Podzamczer D, et al. Durable suppression 2 years after switch to DTG+RPV 2-drug regimen: SWORD 1 and 2 studies [abstract no. THPEB047]. In: 22nd international AIDS conference; 2018.
Revuelta-Herrero JL, Chamorro-de-Vega E, Rodriguez-Gonzalez CG, et al. Effectiveness, safety, and costs of a treatment switch to dolutegravir plus rilpivirine dual therapy in treatment-experienced HIV patients. Ann Pharmacother. 2018;52(1):11–8.
Capetti AF, Cossu MV, Sterrantino G, et al. Dolutegravir plus rilpivirine as a switch option in cART-experienced patients: 96-week data. Ann Pharmacother. 2018;52(8):740–6.
Palacios R, Mayorga M, Gonzalez-Domenech CM, et al. Safety and efficacy of dolutegravir plus rilpivirine in treatment-experienced HIV-infected patients: the DORIVIR study. J Int Assoc Provid AIDS Care. 2018;17:1–4.
Gantner P, Cuzin L, Allavena C, et al. Efficacy and safety of dolutegravir and rilpivirine dual therapy as a simplification strategy: a cohort study. HIV Med. 2017;18(9):704–8.
Diaz A, Casado JL, Dronda F, et al. Dolutegravir plus rilpivirine in suppressed heavily pre-treated HIV-infected patients [abstract no. TUPDB0106 plus poster]. In: 21st international AIDS conference; 2016.
Orkin C, Llibre J, Kahl L, et al. Renal, inflammatory and bone biomarkers following switch to the DTG + RPV 2-drug regimen: the SWORD-1 and SWORD-2 studies [abstract no. O37 plus oral presentation]. HIV Med. 2018;19(Suppl 2):S17.
US FDA. FDA drug safety communication: FDA to evaluate potential risk of neural tube birth defects with HIV medicine dolutegravir (Juluca, Tivicay, Triumeq); 2018. https://www.fda.gov. Accessed 29 Oct 2018.
McComsey GA, Lupo S, Parks D, et al. Switch from tenofovir disoproxil fumarate combination to dolutegravir with rilpivirine improves parameters of bone health. AIDS. 2018;32(4):477–85.
Back D. 2-drug regimens in HIV treatment: pharmacological considerations. Germs. 2017;7(3):113–4.
Boyd MA, Cooper DA. Combination ART: are two drugs as good as three? Lancet. 2018;391(10123):817–9.
Aldir I, Horta A, Serrado M. Single-tablet regimens in HIV: does it really make a difference? Curr Med Res Opin. 2014;30(1):89–97.
Arthurs E, Ward T, Darlington O, et al. Dolutegravir and rilpivirine to treat virologically suppressed adults living with HIV-1: a Canadian cost-utility analysis. Value Health. 2018;21(Suppl 1):S9–10.
Sweet DE, Altice FL, Cohen CJ, et al. Cost-effectiveness of single- versus generic multiple-tablet regimens for treatment of HIV-1 infection in the United States. PLoS One. 2016;11(1):e0147821.
Acknowledgements
During the peer review process, the manufacturer of dolutegravir/rilpivirine was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Hannah Blair is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: D. M. Burger, Radboud University Medical Centre Nijmegen, Nijmegen, Netherlands; J. M. Llibre, HIV Unit, Lluita Contra La SIDA Foundation, Barcelona, Spain; M. Nelson, Chelsea and Westminster Hospital, London, United Kingdom.
Rights and permissions
About this article
Cite this article
Blair, H.A. Dolutegravir/Rilpivirine: A Review in HIV-1 Infection. Drugs 78, 1741–1750 (2018). https://doi.org/10.1007/s40265-018-1005-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-1005-4