Abstract
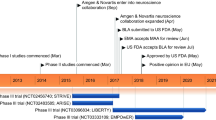
Galcanezumab-gnlm (Emgality™; Eli Lilly and Company), hereafter galcanezumab, is a humanized monoclonal antibody against the calcitonin gene-related peptide (CGRP) ligand. A potent vasodilator, CGRP is implicated in nociceptive transmission and migraine pathogenesis. In September 2018, the US FDA approved galcanezumab as a once-monthly subcutaneous injection for the preventive treatment of migraine in adults. In the same month, the EMA issued a positive opinion for galcanezumab for the prophylaxis of migraine in adults who have at least 4 migraine days per month. Galcanezumab is also undergoing phase III evaluation for the preventive treatment of cluster headache in North America and Europe. This article summarizes the milestones in the development of galcanezumab leading to its first approval for the preventive treatment of migraine in adults.
Similar content being viewed by others
References
Benschop RJ, Collins EC, Darling RJ, et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthr Cartil. 2014;22(4):578–85.
Monteith D, Collins EC, Vandermeulen C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the CGRP binding monoclonal antibody LY2951742 (galcanezumab) in healthy volunteers. Front Pharmacol. 2017;8:740.
Goldberg SW, Silberstein SD. Targeting CGRP: a new era for migraine treatment. CNS Drugs. 2015;29:443–52.
Eli Lilly and Company. Lilly’s Emgality™ (galcanezumab-gnlm) receives U.S. FDA approval for the preventive treatment of migraine in adults [media release]. 5 Oct 2018. http://investor.lilly.com/.
Eli Lilly and Company. EMGALITY (galcanezumab-gnlm) injection, for subcutaneous use: US prescribing information. 2018. http://pi.lilly.com/. Accessed 11 Oct 2018.
European Medicines Agency. Summary of opinion: Emgality (galcanezumab). 2018. http://www.ema.europa.eu/. Accessed 11 Oct 2018.
Eli Lilly and Company. Lilly’s investigational medicine for prevention of migraine met primary endpoint in a Phase 2b study [media release]. 17 June 2015. http://www.lilly.com/.
Eli Lilly and Company. Lilly announces acquisition of CGRP antibody for migraine prevention from Arteaus Therapeutics [media release]. 13 Jan 2014. http://www.lilly.com/.
Atlas Venture. Arteaus Therapeutics, LLC raises $18 M to prevent migraines [media release]. 19 Oct 2011. http://www.atlasventure.com/.
Vermeersch S, Benschop RJ, Van Hecken A, et al. Translational pharmacodynamics of calcitonin gene-related peptide monoclonal antibody LY2951742 in a capsaicin-induced dermal blood flow model. J Pharmacol Exp Ther. 2015;354(3):350–7.
Kielbasa W, O’Brien L, Moser B, et al. Assessment of pharmacokinetics, target engagement and immunogenicity in patients with migraine administered galcanezumab, an anti-CGRP antibody [abstract no. IOR09]. Headache. 2018;58 (Suppl 2):77–8.
Stauffer VL, Dodick DW, Zhang Q, et al. Evaluation of Galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018;75(9):1080–8.
Skljarevski V, Matharu M, Millen BA, et al. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–54.
Aurora S, Detke H, Millen B. Rapid onset of effect of galcanezumab for the prevention of episodic migraine: post-hoc analyses of two phase 3 studies [abstract no. IOR05]. Headache. 2018;58 (Suppl. 2):75.
Detke HC, Wang S, Skljarevski V, et al. A phase 3 placebo-controlled study of galcanezumab in patients with chronic migraine: results from the 3-month double-blind treatment phase of the REGAIN study [abstract no. PS89LB]. Headache. 2017;57(8):1336–7.
Eli Lilly and Company. Summary of the efficacy and safety of galcanezumab in phase 3, randomized, double-blind, placebo-controlled studies. 2017. http://www.headache.mobi/. Accessed 11 Oct 2018.
Eli Lilly and Company. IHC 2017: Lilly’s galcanezumab demonstrates positive long-term safety results for up to 12 months in patients with migraine [media release]. 8 Sep 2017. http://www.lilly.com/.
Skljarevski V, Oakes TM, Zhang Q, et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol. 2018;75(2):187–93.
Eli Lilly and Company. Galcanezumab (LY2951742) CGRP monoclonal antibody for the prevention of migraine: phase 2, randomized, double-blind, placebo-controlled studies, ART01 and CGAB. 2017. http://www.headache.mobi/. Accessed 11 Oct 2018.
Dodick DW, Goadsby PJ, Spierings EL, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13(9):885–92.
Martinez JM, Goadsby PJ, Dodick DW, et al. Study CGAL: a placebo-controlled study of galcanezumab in patients with episodic cluster headache: results from the 8-week double-blind treatment phase [abstract no. 33]. Postgrad Med. 2018;130(Suppl. 1):24–5.
Eli Lilly and Company. Lilly’s galcanezumab meets primary endpoint in phase 3 study evaluating galcanezumab for the prevention of episodic cluster headache [media release]. 15 May 2018. http://www.lilly.com/.
Oakes TMM, Skljarevski V, Zhang Q, et al. Safety of galcanezumab in patients with episodic migraine: a randomized placebo-controlled dose-ranging phase 2b study. Cephalalgia. 2018;38(6):1015–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Galcanezumab: First Global Approval. Drugs 78, 1769–1775 (2018). https://doi.org/10.1007/s40265-018-1002-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-1002-7