Abstract
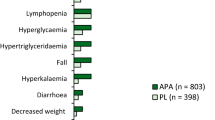
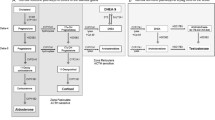
Apalutamide (ErleadaTM) is a next-generation oral androgen receptor (AR) inhibitor that is being developed by Janssen for the treatment of prostate cancer (PC). It binds directly to the ligand-binding domain of the AR and blocks the effects of androgens. In February 2018, apalutamide received its first global approval in the USA for the treatment of non-metastatic castration-resistant PC (nmCRPC). Apalutamide is undergoing phase III investigation in chemotherapy-naive patients with metastatic CRPC (in combination with abiraterone acetate plus prednisone), patients with high-risk localized or locally advanced PC receiving primary radiation therapy, and in patients with metastatic hormone-sensitive PC and biochemically-relapsed PC. This article summarizes the milestones in the development of apalutamide leading to this first approval in nmCRPC.
Similar content being viewed by others
References
Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72(6):1494–503.
US FDA. ErleadaTM (apalutamide): prescribing information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210951s000lbl.pdf. Accessed 26 Mar 2018.
Cancian M, Renzulli JF 2nd. Nonmetastatic castration-resistant prostate cancer: a modern perspective. Urology. 2018. https://doi.org/10.1016/j.urology.2018.01.010.
Rozet F, Roumeguere T, Spahn M, et al. Non-metastatic castrate-resistant prostate cancer: a call for improved guidance on clinical management. World J Urol. 2016;34(11):1505–13.
Janssen Pharmaceutical Companies of Johnsonson & Johnson. ERLEADA (apalutamide), a next-generation androgen receptor inhibitor, granted U.S. FDA approval for the treatment of patients with non-metastatic castration-resistant prostate cancer [media release]. 2018. https://www.prnewswire.com/news-releases/erleada-apalutamide-a-next-generation-androgen-receptor-inhibitor-granted-us-fda-approval-for-the-treatment-of-patients-with-non-metastatic-castration-resistant-prostate-cancer-300598990.html. Accessed 26 Mar 2018.
Janssen Pharmaceutical Companies of Johnsonson & Johnson. Janssen submits marketing authorisation application for apalutamide to treat patients with high-risk non-metastatic castration-resistant prostate cancer [media release]. 2018. https://www.businesswire.com/news/home/20180209005173/en/Janssen-Submits-Marketing-Authorisation-Application-Apalutamide-Treat. Accessed 26 Mar 2018.
Johnson & Johnson. Johnson & Johnson completes acquisition of aragon pharmaceuticals, Inc. [media release]. 2013. https://www.jnj.com/media-center/press-releases/johnson-johnson-completes-acquisition-of-aragon-pharmaceuticals-inc. Accessed 26 Mar 2018.
Koukourakis MI, Kakouratos C, Kalamida D, et al. Comparison of the effect of the antiandrogen apalutamide (ARN-509) versus bicalutamide on the androgen receptor pathway in prostate cancer cell lines. Anti Cancer Drugs. 2018. https://doi.org/10.1097/CAD.0000000000000592.
Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3(9):1020–9.
Rathkopf DE, Smith MR, Ryan CJ, et al. Androgen receptor mutations in patients with castration-resistant prostate cancer treated with apalutamide. Ann Oncol. 2017;28(9):2264–71.
Rathkopf DE, Morris MJ, Fox JJ, et al. Phase I study of ARN-509, a novel antiandrogen, in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2013;31(28):3525–30.
Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018. https://doi.org/10.1056/NEJMoa1715546.
Smith MR, Antonarakis ES, Ryan CJ, et al. Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur Urol. 2016;70(6):963–70.
Rathkopf DE, Antonarakis ES, Shore ND, et al. Safety and antitumor activity of apalutamide (ARN-509) in metastatic castration-resistant prostate cancer with and without prior abiraterone acetate and prednisone. Clin Cancer Res. 2017;23(14):3544–51.
Chi KN, Chowdhury S, Radziszewski P, et al. TITAN: a randomized, double-blind, placebo-controlled, phase 3 trial of apalutamide (ARN-509) plus androgen deprivation therapy (ADT) in metastatic hormone-sensitive prostate cancer (mHSPC) [abstract no. 771TiP]. Ann Oncol. 2016;27(Suppl. 6):vi243–65.
Rathkopf DE, Attard G, Efstathiou E, et al. A phase 3 randomized, placebo-controlled double-blind study of ARN-509 plus abiraterone acetate (AA) in chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC) [abstract no. TPS5071]. J Clin Oncol Conf. 2015;33(15 Suppl.).
McKenzie M, Dearnaley D, Tombal B, et al. ATLAS: a randomized, double-blind, phase 3 study of ARN-509 in patients with high-risk localized or locally advanced prostate cancer receiving primary radiation therapy [abstract no. MP-07.10]. Can Urol Assoc J. 2016;10(5-6 Suppl. 1):S73–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered and accepted an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Zaina T. Al-Salama is a salaried employee of Adis/Springer, is responsible for the article content, and declares no relevant conflicts of interest.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Al-Salama, Z.T. Apalutamide: First Global Approval. Drugs 78, 699–705 (2018). https://doi.org/10.1007/s40265-018-0900-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0900-z