Abstract
An intramuscular formulation of onabotulinumtoxinA (onabotA; Botox®) is currently the only therapy specifically approved for the prevention of headaches in adults with chronic migraine (CM) in the EU and North America. This article provides a narrative review of relevant data on the drug in this indication from an EU perspective. OnabotA was originally approved on the basis of pooled data from two phase III studies (PREEMPT 1 and 2). In these pivotal studies, injection of up to five cycles of onabotA (155–195 U/cycle) at 12-week intervals was generally well tolerated and effective in producing statistically significant and clinically meaningful improvements in headache symptoms, acute headache pain medication usage, headache impact and health-related quality of life in adults with CM, of whom approximately two-thirds were acute medication overusers and approximately one-third had failed to respond to ≥ 3 prior oral prophylactic therapies. More recently, the efficacy and tolerability of onabotA over a period of 1 year in the PREEMPT programme has been substantiated and extended by the results of a long-term phase IV study (COMPEL), in which patients received up to nine treatment cycles over a period of 2 years, and by findings from several real-world clinical practice studies from Europe, including the prospective multinational REPOSE and CM-PASS studies. In conclusion, the totality of evidence from clinical trials and real-world studies indicates that onabotA is an effective and generally well tolerated option for the prevention of CM that may be particularly useful for patients who have previously failed to respond to or are intolerant of commonly prescribed oral prophylactics.
Similar content being viewed by others
Only therapy specifically approved for the prevention of headaches in adults with CM in the EU |
Therapy involves regular (3-monthly) injections |
Efficacy and tolerability in large clinical trials confirmed in large real-world studies |
Beneficial in patients regardless of whether or not they are acute medication overusers |
Neck pain, (facial-) muscle weakness and eyelid ptosis are the most common treatment-related adverse events |
1 Introduction
Migraine, a disabling neurological disorder characterized by severe headache and associated symptoms (e.g. nausea, vomiting, photophobia and/or phonophobia) [1], exists along a continuum between episodic and chronic (i.e. more frequent, severe and burdensome) forms of the disease [2]. According to the current International Headache Society classification of headache disorders (ICHD-3), chronic migraine (CM) is defined as headache on ≥ 15 days per month for > 3 months, of which ≥ 8 days meet the criteria for migraine with or without aura and/or respond to migraine-specific treatment, occurring in a patient with a history of at least five prior migraine attacks not attributed to another causative disorder or medication overuse [1]. It affects ≈ 1–2% of the general population [3] and usually evolves from episodic migraine (EM; defined as < 15 headache days per month) [4], with chronification occurring in ≈ 3% of patients with EM annually [5]. CM is classified as a distinct clinical entity [6]; patients with CM experience more headache days, but also increased headache-related disability, reduced health-related quality of life (HR-QOL) and greater co-morbidity than those with EM [7]. CM is associated with higher healthcare resource utilization and socioeconomic burden compared with EM [6, 7]. Historically, only a minority of patients with CM have been correctly diagnosed [8]; however, a simple case-finding tool that accurately identifies most patients with CM has recently been developed and validated [Identify Chronic Migraine (ID-CM)] [9].
The management of CM centres around three main approaches: lifestyle modification and behavioural therapy; use of acute medications to relieve or ameliorate the symptoms of a migraine attack that has already begun; and the use of preventative pharmacotherapies to reduce the frequency, duration and severity of attacks, thereby limiting the need for acute medications, as these may be causing concurrent medication overuse headache (MOH) [10, 11]. Various oral agents, including β-adrenoreceptor antagonists and anticonvulsants, have been shown to be effective in the prevention of migraine in general [12, 13] and are recommended for use as first- or second-line therapies by EU guidelines [14,15,16]. However, none of these agents are specifically licenced for the prevention of CM in this region. Moreover, they have shortcomings in terms of efficacy, tolerability and adherence; additional pharmacological and non-pharmacological interventions have been investigated in response to the long-identified need for more effective therapies for patients with CM [11, 17].
OnabotulinumtoxinA (hereafter referred to as onabotA) [Botox®], a formulation of botulinum toxin type A (BoNT/A) administered by intramuscular injection, is currently the only therapy specifically approved for the prevention of CM (i.e. headaches in adults with CM) in the EU [18] and North America [19, 20]. This article briefly summarizes the pharmacological properties of onabotA and, from an EU perspective, provides a narrative review of data from clinical trials and real-world studies pertaining to its efficacy and tolerability in the prevention of CM. The developmental history of onabotA for CM has been described elsewhere [21].
2 Pharmacological Properties of OnabotulinumtoxinA
The pharmacodynamic properties of onabotA include a well characterized temporary muscle relaxant effect, which results from the toxin entering motor nerve terminals and cleaving nine amino acids from the C-terminus of the Soluble NSF-Attachment Protein Receptor (SNARE) protein SNAP25 (SNAP25206) to yield SNAP25197, thereby disrupting exocytosis and blocking neurotransmitter release [22].
The exact mechanism of action whereby extracranial administration of onabotA prevents headaches in patients with CM is being elucidated [21, 23].
The most widely promulgated notion is founded on the phenomena of central and peripheral sensitization within the trigeminovascular system, both of which have been implicated in the pathophysiology of migraine/CM [21, 24,25,26,27]. According to this theory, injection of onabotA in the trigeminally-innervated cranio-facial-cervical region blocks peripheral sensitization as a result of inhibiting the release of pain-mediating peptides, especially calcitonin gene-related protein (CGRP), from peripheral nociceptive neurones; this reversal of peripheral sensitization leads indirectly to reversal of central sensitization [21, 24, 28, 29]. In addition to inhibiting the release of pain-mediating peptides, onabotA may reduce peripheral sensitization by interfering with the integration of relevant sensory receptors and ion channels [e.g. transient receptor potential cation channel vanilloid subfamily member 1 (TRPV1) and transient receptor potential cation channel ankyrin subfamily member 1 (TRPA1)] on nociceptive nerve endings [21, 25, 30]. Both actions are thought to involve inhibition of SNARE-mediated synaptic vesicle trafficking by onabotA [21].
An alternative hypothesis, namely that onabotA exerts a direct effect on central pain processing as a result of retrograde transport in peripheral nociceptive neurones and transcytosis to second-order neurones [31], has received support from an in vitro study that examined the trafficking of clostridial neurotoxins (including onabotA) in central neurones grown in microfluidic devices [32]. However, an in vivo study that used a highly selective antibody for SNAP25197 combined with 3-dimensional imaging and quantitative analysis found no evidence in favour of transcytosis [33]. Under the prevailing experimental conditions, onabotA was confined to primary motorneurones following peripheral administration in rats; any suggestion of distal activity was due to limited systemic spread of the toxin at higher doses [33].
Interestingly, distinct structural and functional brain changes have been observed in patients with CM who respond to prophylactic therapy with onabotA (i.e. revert to EM) compared with those who do not respond to therapy [34].
The pharmacokinetics of onabotA have not been studied due to the neurotoxic nature of the product. However, little systemic absorption of onabotA is believed to occur following intramuscular injection of therapeutic doses; it is probably metabolised by proteases and the molecular components recycled through normal metabolic pathways [35]. Like other BoNT/A products, onabotA exhibits a low immunogenic potential [36], as exemplified by the fact that none of 496 analysable patients with EM or CM had a confirmed positive test for neutralizing antibodies after up to three 12-week treatment cycles in phase II studies [37].
3 Therapeutic Efficacy of OnabotulinumtoxinA
In terms of key clinical trials, the efficacy of injected onabotA for headache prevention in adults with CM has been evaluated over a period of 1 year in the pivotal phase III PREEMPT 1 and 2 studies (Sect. 3.1) and, longer-term, over a period of 2 years in the phase IV COMPEL study (Sect. 3.2). In addition, a number of real-world studies have examined the effectiveness of onabotA for the prevention of CM in routine clinical practice in Europe, typically for periods of up to 2 years (Sect. 3.3).
3.1 PREEMPT Trials
The multicentre PREEMPT 1 [38] and 2 [39] trials together comprised the PREEMPT clinical trial programme. Apart from the designation of the primary and secondary endpoints (discussed in detail elsewhere [17]), these studies were identical in design; their findings have been analysed separately [38, 39] or pooled [37, 40,41,42,43,44].
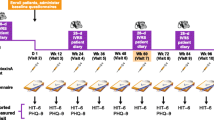
Briefly, both studies consisted of a 24-week randomized, double-blind, placebo-controlled phase followed by a 32-week open-label extension phase [38, 39]. Eligible patients were men and women aged 18–65 years with CM defined according to the then-prevailing (2004) ICHD-2 criteria [45]. To enter the placebo-controlled phase, they were required to have had (1) ≥ 15 headache days (with each day consisting of ≥ 4 h of continuous headache and with ≥ 50% of days being migraine or probable migraine days) and (2) at least four distinct headache episodes (with each episode lasting ≥ 4 h) during a 28-day (i.e. 1 month) baseline period prior to randomization; patients were stratified according to whether or not they met the protocol definition for overuse of acute headache pain medications during this period [38, 39].
OnabotA was injected into 31 sites across seven specific head/neck muscle areas (frontalis, corrugator, procerus, occipitalis, temporalis, trapezius and cervical paraspinal) using a fixed-dose/fixed-site approach (5 U per injection; one injection per site). Up to eight additional injections (each of 5 U) could be administered in up to three specific muscle areas (occipitalis, temporalis and trapezius) using a ‘follow-the-pain’ strategy. Hence, the minimum/maximum total dose of onabotA per treatment cycle was 155 U into 31 sites/195 U into 39 sites; this injection pattern is hereafter referred to as the ‘PREEMPT injection protocol’ [46] (Sect. 5). Study medications were administered at 12-week intervals; patients underwent up to five treatment cycles: two with onabotA or placebo during the double-blind phase; and three with onabotA during the open-label phase [38, 39].
A combined total of 1384 patients were initially randomized to receive onabotA (n = 688) or placebo (n = 696) in the two trials [40]. The majority of participants were female (86%) and Caucasian (90%); they had an average of 20 headache days per month at baseline [40]. Approximately two-thirds (65%) were overusing acute headache pain medications at baseline [40]. Approximately two-fifths (42%) had previously tried a first-line migraine prophylactic (according to UK guidelines [15]) [37]; approximately one-third (35%) had previously failed to respond to ≥ 3 preventative therapies [47].
Patient demographics and disease characteristics at baseline were mostly balanced between the treatment groups in the individual studies [38, 39]. In PREEMPT 1, however, patients allocated to onabotA had significantly fewer headache (12.3 vs 13.4 per month; p = 0.023) and migraine (11.5 vs. 12.7 per month; p = 0.006) episodes than those assigned to placebo, and significantly more cumulative hours of headache on headache days (295.7 vs. 274.9 h; p = 0.022) [38]. These between-group differences were reflected in the pooled analyses of the two trials [40, 42].
3.1.1 Results
Key findings from the 24-week, double-blind phases of PREEMPT 1 and 2 are presented in Table 1. Notably, both studies were characterized by a large placebo response [38,39,40]; onabotA achieved a statistically significant improvement versus placebo for the primary endpoint in PREEMPT 2 (mean change from baseline to week 24 in the monthly frequency of headache days), but not the primary endpoint in PREEMPT 1 (mean change from baseline to week 24 in the monthly frequency of headache episodes) (Table 1) [38].
According to a pooled analysis of the two trials, the mean decrease from baseline to week 24 in the monthly frequency of headache days (the primary endpoint) significantly favoured onabotA over placebo (Table 1) [40]. Indeed, based on pooled data, mean changes from baseline in the monthly frequency of headache days, moderate to severe headache days, severe headache days, headache episodes, migraine days and migraine episodes significantly (p ≤ 0.027) favoured onabotA over placebo at all time points through week 24, as did the monthly cumulative hours of headache on headache days [40, 42].
Other pooled analyses indicated that, at week 24, significantly (p < 0.001) more onabotA than placebo recipients demonstrated a clinically meaningful ≥ 50% decrease from baseline in the monthly frequency of headache days (47.1 vs. 35.1%), moderate to severe headache days (49.4 vs. 37.5%) and migraine days (48.2 vs. 36.4%) as well as in the monthly cumulative hours of headache on headache days (50.3 vs. 38.9%) [41]; additionally, significantly (p < 0.001) more onabotA recipients experienced an improvement in headache-day severity [i.e. ≥ 1-grade improvement from baseline in Average Daily Headache Severity (ADHS) score: 35.8 vs. 23.1%] [42].
Moreover, a pooled subgroup analysis [43] indicated that patients who experienced an inadequate reduction in headache-day frequency nonetheless experienced a reduction in headache-day severity. Specifically, in the subset of patients who demonstrated a < 50% decrease from baseline in the monthly frequency of headache days (n = 645), the mean decrease from baseline to week 24 in the monthly frequency of severe headache days significantly (p = 0.001) favoured onabotA over placebo (1.8 vs. 0.4), as did the proportion of severe headache days per month (30.3 vs. 35.5%); significantly (p = 0.01) more onabotA recipients experienced a ≥ 1-grade improvement from baseline in ADHS score (41.1 vs. 31.4%; p = 0.01) [43].
In other pooled subgroup analyses, statistically significant (p < 0.05) reductions in all headache symptoms favouring onabotA over placebo were seen among patients who were medication overusers at baseline [44, 48] and those who had previously tried a first-line migraine prevention medication [37]. Generally similar results were seen among patients who were not medication overusers at baseline [44] or had not previously tried a first-line prophylactic medication [37]. Among patients who had previously failed to respond to ≥ 3 preventative therapies, the reduction from baseline in the monthly frequency of headache days was significantly greater among onabotA recipients (n = 231) compared with placebo recipients (n = 248) [7.4 vs. 4.7 days; p < 0.001] [47].
As regards assessments of acute medication use, onabotA therapy was associated with significant reductions in the number of acute headache pain medication intake days per month and as well as in the monthly frequency of triptan intakes, based on pooled PREEMPT data (Table 1) [41].
OnabotA therapy was also associated with a significant reduction in headache impact, as assessed using the Headache Impact Test-6 (HIT-6), and a significant improvement in HR-QOL, as assessed using the Migraine Specific Quality of Life Questionnaire (MSQ), based on pooled PREEMPT data (Table 1) [38, 39]. Specifically, statistically significant and clinically meaningful reductions in total HIT-6 score favouring onabotA over placebo were seen at all assessments from week 16 through week 24, while statistically significant and clinically meaningful improvements in all three MSQ domain scores favouring onabotA over placebo were seen at both time points assessed, namely week 12 and week 24 [49, 50]. In pooled subgroup analyses, statistically significant and clinically important reductions in headache impact and improvements in HR-QOL favouring onabotA over placebo were seen regardless of whether or not patients were medication overusers [48] and, in general, irrespective of whether or not they had received prior first-line prophylactics [37].
Based on pooled analyses, all assessments of headache symptoms, acute medication use, headache impact and HR-QOL continued to improve relative to baseline during the 32-week, open-label extension phase (when all patients were treated with onabotA), both in patients who had previously received onabotA during the double-blind phase (‘onabotA-only group’) and those who had previously received placebo (‘placebo/onabotA group’) [41, 50] (Table 1). Significant improvements from baseline to week 56 were observed for all of these assessments, both in the onabotA-only group and the onabotA/placebo group [41, 50]. However, some assessments of headache symptoms (e.g. headache-day frequency) and HR-QOL (i.e. the MSQ role restrictive domain) still significantly favoured the onabotA-only group over the onabotA/placebo group at the end of the open-label phase (Table 1) [41, 50]. A pooled subgroup analysis of patients who completed all five treatment cycles (n = 513 and 492 in the onabotA-only and placebo/onabotA groups, respectively) yielded mostly similar results, further supporting the suggestion that, at week 56, patients who started onabotA therapy earlier in these trials had better outcomes than those who began therapy later [41, 51].
Importantly, a post hoc pooled analysis provided evidence that a meaningful proportion of patients who did not respond after the first onabotA treatment cycle did respond after the second or third cycle [52]. In terms of achieving a ≥ 50% decrease from baseline in the monthly frequency of headache days, 78 (22%) of the 349 onabotA-treated patients who did not reach this endpoint after the first cycle did demonstrate a ≥ 50% decrease after the second cycle (Fig. 1). Furthermore, 71 (26%) of the 271 onabotA-treated patients who did not demonstrate a ≥ 50% decrease in the monthly frequency of headache days after the first and second cycles did reach this endpoint after the third cycle (Fig. 1). Similar results were seen for other key outcomes (Fig. 1) [52].
Proportion of onabotulinumtoxinA-treated patients who responded (with a ≥ 50% improvement from baseline in the headache symptom or impact assessment indicated) for the first time after treatment cycles 1, 2 and 3 in the pooled PREEMPT trials [52]. For each assessment, the maximum possible number of first-time responders in the cycle indicated is shown above the bar. HA headache, HIT-6 Headache Impact Test-6
3.2 COMPEL Study
In the multinational, open-label COMPEL study [53,54,55,56,57,58,59,60,61,62], adults with CM (ICHD-2 criteria [45]) received up to nine onabotA treatment cycles (155 U/12-week cycle; administered as per the PREEMPT injection protocol); the design of this study is described in detail elsewhere [54]. Consistent with the PREEMPT studies, most of the 715 evaluable participants were female (85%) and Caucasian (81%); they reported an average of 22 headache days per month at baseline. Just over one-half (52.1%) completed all nine treatment cycles (108 weeks) [53].
Clinical benefits persisted during long-term (2 years) onabotA therapy. Regarding the monthly frequency of headache days, there was a significant reduction relative to baseline at week 108 (−10.7 days; p < 0.0001) [primary outcome]; sequential improvements were seen at all earlier assessments [− 7.4, −9.2 and − 9.8 days after 2, 5 and 7 cycles (24, 60 and 84 weeks), respectively; all p < 0.0001 vs. baseline] [53].
Assessments of headache impact (total HIT-6 score) [53], migraine-related disability (Migraine Disability Assessment Questionnaire) [56, 57], HR-QOL (all three MSQ domain scores) [56, 57], sleep disturbance (Pittsburgh Sleep Quality Index) [58], fatigue (Fatigue Severity Scale) [58], depression (9-item Patient Health Questionnaire) [59] and anxiety (7-item Generalized Anxiety Disorder Assessment) [59] were also significantly (p < 0.0001) improved from baseline at week 108; similar improvements were seen at all earlier assessments [56,57,58,59].
Consistent with the overall study population, significant (p < 0.0001 vs. baseline) improvements in headache frequency, migraine-related disability and HR-QOL assessments were seen at all time points in subgroup analyses of patients with headaches every day [61, 62] or allodynia [60, 62] at baseline.
Despite differences in study design, the patient population in COMPEL was, as noted above, similar to that in the PREEMPT trials, and outcomes at week 24 were generally consistent, based on a descriptive comparison of COMPEL and pooled PREEMPT data [55].
3.3 Real-World Studies
From among the many investigations that have demonstrated the effectiveness of onabotA as a preventative therapy for CM in real-world clinical practice in Europe, this section focuses on findings from four prospective, observational studies that each enrolled > 150 patients at one or more treatment centres throughout the region [63,64,65,66,67,68,69,70,71,72,73].
Of note, the post-authorization REPOSE study is the only one of these trials to have been conducted across multiple countries [70,71,72]. Briefly, among 641 patients enrolled at 78 centres in Germany, Italy, Norway, Russia, Spain, Sweden and the UK, 633 (85% of whom were women) received at least one onabotA dose for a total of 3499 treatment sessions over a period of 2 years [72]. OnabotA was administered at ≈ 3-month intervals as per the physician’s usual practice, guided by the summary of product characteristics; the majority of treatment sessions were consistent with the PREEMPT protocol [72].
Consistent with the results of clinical trials (PREEMPT and COMPEL), onabotA therapy significantly (p < 0.001) reduced the mean monthly frequency of headache days relative to baseline; sequential improvements were seen at all assessments (e.g. − 8.2, − 9.1, − 11.4, − 13.0 and − 13.3 days after 1, 2, 4, 6 and 8 cycles, respectively) [72]. Regarding HR-QOL, all three MSQ domain scores increased significantly (p < 0.001) from baseline at both time points assessed, namely after cycle 2 and cycle 8 [72]. Similar improvements in headache symptoms and HR-QOL (based on MSQ and EQ-5D total scores) were seen in both the 6-month [70] and 1-year [71] REPOSE study interim analyses.
Patients enrolled in the remaining studies (from single centres in Italy [63, 64] and the UK [65,66,67,68,69] or from 13 centres in Spain [73]) had failed to respond to or were intolerant of ≥ 1 oral preventative therapy [63,64,65, 73]; around one-half [65, 68, 69, 73] or all [63, 64] of them fulfilled criteria for medication overuse [63,64,65, 68, 69, 73] or had comorbid MOH [63, 64] at baseline. The majority (78–86%) were women [64, 65, 73].
Prophylactic treatment with onabotA administered in accordance with the PREEMPT protocol significantly (p < 0.01 vs. baseline) improved a range of headache symptom and impact measures, as assessed after, for example, 1 cycle [63,64,65, 73] or 1 [73] or 2 [63, 64] years of treatment. Similar to REPOSE [72], these assessments showed progressive improvements during 1 [73] or 2 [63, 64] years of therapy.
An updated and expanded analysis from the UK study [68] (n = 434 vs. 254 [65]) showed that onabotA was equally effective in patients with or without medication overuse at baseline. In terms of achieving a clinically meaningful response, 32% of patients reported a ≥ 50% reduction in headache-day frequency (PREEMPT criteria), 47% reported a ≥ 30% reduction in headache-day frequency [National Institute for Health and Care Excellence (NICE) criteria] and 66% reported a ≥ 50% reduction in headache- or migraine- day frequency or an increase in headache-free days twice that of baseline (Hull Headache Clinic criteria) after the first treatment cycle [65]. Another updated and expanded analysis of this study [69] (n = 536) showed that patients with or without medication overuse responded equally well to the first treatment cycle.
Interesting information on long-term outcomes in patients achieving a clinically meaningful response is also available from the UK study [67]. Patients who were responders after two cycles (NICE or Hull criteria) and continued to a third cycle were allowed to stop therapy if they experienced < 10 headache days per month for 3 consecutive months (Hull modified positive stopping rule); 56 (61.5%) of 91 initial responders who were able to successfully stop therapy within 2 years continued to show a sustained response. However, 17 (19%) patients relapsed and restarted therapy; overall 84 (48%) of 175 initial responders were still receiving onabotA at 2 years. Of note, 11 (12%) of 91 patients became resistant and reverted to CM while still receiving onabotA [67].
4 Tolerability of OnabotulinumtoxinA
Repeated injection of onabotA (155–195 U) every 12 weeks for up to five cycles was generally well tolerated in the PREEMPT clinical trial programme discussed in Sect. 3.1 [38,39,40,41, 51]. OnabotA recipients mostly reported adverse events (AEs) that were mild or moderate in severity and resolved without sequelae; they infrequently discontinued therapy due to AEs [3.8 (vs. 1.2% of placebo recipients) during the double-blind phase of the pooled PREEMPT studies; 2.6% during the open-label extension phase of the pooled PREEMPT studies] [40, 41].
Moreover, treatment-related AEs (TRAEs) were consistent with the known tolerability profile of onabotA when injected into head and neck muscles; no new safety events were observed [40, 41, 51]. The overall rates of TRAEs were 29.4% for patients receiving two cycles of onabotA during the double-blind phase (n = 687) [vs. 12.7% for placebo (n = 692)] [40] and 34.8% for patients receiving all five cycles of onabotA during the double-blind and open-label extension phases (n = 515) [51]. Of note, the rate of TRAEs decreased progressively with each subsequent treatment session, being 48.3, 37.2, 37.8, 26.3 and 19.1% after the first, second, third, fourth and fifth cycles of onabotA, respectively [51]. The most commonly observed TRAEs in patients receiving onabotA included neck pain [6.7 (vs. 2.2% with placebo) during the double-blind phase; 4.6% during the open-label extension phase of the pooled PREEMPT studies], muscle weakness [5.5 (vs. 0.3%); 3.9%], eyelid ptosis [3.3 (vs. 0.3%); 2.5%], injection-site pain [3.2 (vs. 2.0%); 2.0%] and musculoskeletal pain [2.2 (vs. 0.7%); 1.1%] [41]. Facial paresis accounted for two-fifths of the reports of muscle weakness during the double-blind phase (incidence of 2.2%) and for nearly one-third of the reports of muscle weakness during the open-label extension phase (incidence of 1.2%) [41]. Only one onabotA recipient in the pooled PREEMPT studies experienced a serious TRAE (migraine requiring hospitalization) [39].
Design differences notwithstanding, tolerability findings from the PREEMPT trials are supported and extended by those of the long-term, open-label COMPEL study [53] (Sect. 3.2) and, where reported, several real-world studies [63,64,65, 70, 71] (Sect. 3.3). In COMPEL, for example, the overall rate of TRAEs was 15.0% at week 24 [55] and 18.3% at week 108 [53]. The most frequently reported TRAE was neck pain, both at week 24 (occurring in 2.9% of patients) [55] and week 108 (4.1%) [53]. As in the PREEMPT programme, only one patient in COMPEL reported a serious TRAE (rash); no novel safety signals were seen [56].
To date, the largest completed study to evaluate the safety of onabotA for the preventative treatment of CM in routine clinical practice has been a prospective, observational, multinational, post-authorization study (hereafter referred to as ‘CM-PASS’) [74, 75]. Briefly, the study population consisted of 1160 patients (84% women; 98% Caucasian) enrolled at 58 centres across Germany, Spain, Sweden and the UK. The majority (86%) had a diagnosis of CM or transformed (i.e. chronified) migraine at baseline; approximately one-quarter (24.7%) were medication overusers. Almost one-half (43.9%) were receiving ≥ 1 acute and ≥ 1 preventative therapy at baseline; approximately one-half (51%) had previously received onabotA for CM [74]. Participating physicians were provided with the summary of product characteristics, but were not mandated to follow the PREEMPT injection protocol set out therein. Most (90.1%) patients underwent ≥ 1 treatment session that deviated from the recommended label treatment paradigm, although the median dose (155 U) and median number of injection sites (n = 31) were consistent across all observed onabotA treatment sessions (n = 4017) and in line with the PREEMPT protocol. The median interval between sessions was 13.7 weeks [74].
Over a period of 64 weeks, one-quarter (25.1%) of CM-PASS participants reported ≥ 1 TRAE, most frequently neck pain (4.4%) and eyelid ptosis (4.1%). TRAEs of special interest included worsening of migraine (4.0%), intractable migraine (0.4%) and dysphagia (0.3%) [74]; the incidence rates of intractable migraine and dysphagia (secondary and primary outcome measures, respectively) were 1.6 and 0.4 per 1000 person-months [75]. As in the PREEMPT and COMPEL studies, only one patient reported a serious TRAE (worsening of migraine) [74]. Of note, approximately three-quarters (74.4%) of 1090 evaluable patients indicated they were satisfied/extremely satisfied with onabotA therapy; this included, respectively, 83 and 65% of patients who had and had not previously received onabotA [74].
5 Dosage and Administration of OnabotulinumtoxinA
In the EU, onabotA has been approved for the prevention of headaches in adults with CM through the mutual recognition procedure, with Ireland as the reference state [18]. However, the exact wording of the indication may vary between member states, and local prescribing information should be consulted for specific details.
As per the PREEMPT clinical protocol, the recommended dose of onabotA is 155–195 U administered intramuscularly as 0.1 mL (5 U) injections to 31 and up to 39 sites across seven specific head/neck muscle areas as follows: corrugator [10 U (2 sites)]; procerus [5 U (1 site)]; frontalis [20 U (4 sites)]; temporalis [40 U (8 sites) up to 50 U (up to 10 sites)]; occipitalis [30 U (6 sites) up to 40 U (up to 8 sites)]; cervical paraspinal muscle group [20 U (4 sites)]; and trapezius [30 U (6 sites) up to 50 U (up to 10 sites)] [35]. All muscles should be injected bilaterally, with the exception of the procerus, which should be injected at one site only (midline). The recommended retreatment schedule is every 12 weeks [35].
Local prescribing information should be consulted for full details of dosage and administration guidelines, contraindications and warnings and precautions relating to the use of onabotA for the prevention of CM.
6 Place of OnabotulinumtoxinA in the Prevention of Chronic Migraine
The pivotal PREEMPT programme showed that treatment with up to five cycles of onabotA (155–195 U/cycle) at 12-week intervals was generally well tolerated (Sect. 4) and effective in reducing headache symptoms, headache impact and acute headache pain medication usage, as well as improving HR-QOL, in patients with CM, approximately two-thirds of whom were medication overusers and approximately one-third of whom had failed to respond to ≥ 3 prior preventative therapies (Sect. 3.1). Improvements in headache symptoms, headache impact and HR-QOL favouring onabotA over placebo were seen regardless of whether or not patients were medication overusers and, in general, irrespective of whether or not they had received prior first-line prophylactics (Sect. 3.1). Moreover, onabotA not only reduced headache-day frequency, but also headache-day severity; a reduction in headache-day severity was seen even in patients who did not experience a clinically meaningful (i.e. ≥ 50%) reduction in headache-day frequency (Sect. 3.1).
During the open-label phase, in which all patients received three cycles of onabotA, all assessments of headache symptoms, acute medication usage, headache impact and HR-QOL continued to improve relative to baseline (Sect. 3.1). Further evidence of the benefit over time of repeated administration of onabotA exists in the form of the observation that around one-quarter of those patients who did not achieve a clinically meaningful (i.e. ≥ 50%) reduction in headache symptoms after the first treatment cycle did respond after the second cycle, while approximately one-third of those patients who did not achieve a clinically meaningful reduction in headache impact after the first treatment cycle did respond after the second cycle (Sect. 3.1). Moreover, around one-quarter of those patients who did not respond in terms of these endpoints after the first and second cycles did respond after the third cycle (Sect. 3.1). This suggests, therefore, that in practice at least two to three cycles of onabotA should be attempted before categorizing those patients who do not respond initially as nonresponders [52].
Debate surrounding the PREEMPT studies has centred on the small treatment effect of onabotA relative to placebo, the possibility that blinding was inadequate and the relevance of the evaluated population [17, 27]. The totality of data from the PREEMPT programme has, nonetheless, led to onabotA becoming the first (and so far only) headache prophylactic therapy to be specifically approved for CM in the UK (Sect. 1). Importantly, results from the PREEMPT programme are specific to onabotA and cannot be extrapolated to other commercially available formulations of botulinum toxin A, namely abobotulinumtoxinA (Dysport™) and incobotulinumtoxinA (Xeomin®).
The current European Federation of Neurological Societies guideline on the pharmacological treatment of migraine predates the approval of onabotA and does not mention the drug [16]. As regards national guidelines, onabotA has a level 1 (highest) recommendation for the preventive treatment of CM in Italy [76]. In the UK, both NICE [47] and the Scottish Medicines Consortium [77] recommend that onabotA be reserved for patients with CM (i.e. headaches on ≥ 15 days per month of which ≥ 8 days meet the criteria for migraine) who have failed to respond to ≥ 3 prior preventative therapies and whose condition is appropriately managed for medication overuse.
Key findings for onabotA over a period of 1 year in the PREEMPT programme have been substantiated and extended by the results of a 2-year clinical study (COMPEL; Sect. 3.2) and several large real-world studies from Europe, including REPOSE (Sect. 3.3) and CM-PASS (Sect. 4). These studies have generally enrolled patients similar to those who participated in PREEMPT and have variously confirmed the efficacy and safety of short and longer-term prevention with onabotA administered as per the PREEMPT protocol or, in the case of the multinational REPOSE [74] and CM-PASS [78, 79] studies, largely administered in line with this injection paradigm. In COMPEL, sustained benefits were seen in patients who received up to nine treatment cycles (Sect. 3.2), while in real-world studies, benefits have been seen in patients who have failed to respond to or are intolerant of prior oral preventative therapies, including those with concomitant medication overuse or comorbid MOH (Sect. 3.3). In addition, a small real-world from Europe [80] has reported a delayed and progressively beneficial effect among patients who continue to receive treatment after failing to respond to the first cycle; this supports the notion that at least another one to two cycles should be attempted before deeming those individuals who do not respond initially to be nonresponders [81].
Data are also emerging from real-world studies that address current areas of uncertainty surrounding the use of onabotA for the prevention of CM, including how to identify patients who are more (or less) likely to respond, the appropriate duration of therapy in responders, the rate of sustained benefit in patients who successfully stop therapy (positive stopping rule), and the rate of relapse in patients who stop therapy. Duration of disease [73], unilaterality of pain [73], intensity of headache [73], interictal CGRP levels [82] and (in women) polymorphisms in genes encoding CGRP [83, 84] and TRPV1 [84] are among the potential predictors of efficacy that have been identified, based on data collected from clinical practice in Spain [73, 82,83,84] and the UK [85]. Among actual responders, the pattern of response appeared to be predictive of longer-term outcome [66]. Based on 2 years’ follow-up at one UK centre [67], nearly two-thirds of patients who successfully stopped therapy showed sustained benefit, while approximately one-fifth relapsed. Overall, approximately one-half of initial responders were still receiving therapy at 2 years (Sect. 3.3). Issues relevant to optimizing the long-term management of CM with onabotA in real-world clinical practice, such as when to initiate therapy and how to define (and appropriately treat) responders and nonresponders, are discussed in more detail elsewhere [81].
On the basis of pharmacoeconomic analyses that incorporate data from the PREEMPT trials, the use of onabotA for the prevention of CM can be considered cost-effective from the perspective of the National Health Service in Italy [86] and the UK [87]. The real-world cost-effectiveness of onabotA in clinical practice in Europe remains to be determined, although relevant data regarding healthcare resource utilization (HRU) are being collected as part of the multinational REPOSE study [88]. In this regard, interim (1-year) data from participating centres in Germany indicated that use of onabotA not only reduced HRU (e.g. physician visits and technical investigations), but also improved work performance and disability [88].
In conclusion, the totality of evidence from clinical trials and real-world studies indicates that onabotA is an effective and generally well tolerated option for the prevention of CM that may be particularly useful for patients who have previously failed to respond to or are intolerant of commonly prescribed oral prophylactics.
Data Selection OntabotulinumtoxinA: 300 records identified
Duplicates removed | 51 |
Excluded at initial screening (e.g. press releases; news reports; not relevant drug/indication) | 23 |
Excluded during initial selection (e.g. preclinical study; reviews; case reports; not randomized trial) | 35 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 110 |
Cited efficacy/tolerability articles | 39 |
Cited articles not efficacy/tolerability | 42 |
Search Strategy: EMBASE, MEDLINE and PubMed from 2012 to present. Previous Adis Drug Evaluation published in 2012 was hand-searched for relevant data. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Onabotulinumtoxin-A, Onabot-A, Botox, BoNT-A, AGN-191622, botulinum, onabotulinum, neurotoxin A. Records were limited to those in English language. Searches last updated 16 February 2018. | |
Change history
20 April 2018
The article OnabotulinumtoxinA: A Review in the Prevention of Chronic Migraine, written by James E. Frampton and Stephen Silberstein, was originally published Online First without open access. After publication in volume 78, issue 5, pages 589–600 [funder] requested that the article be Open Choice to make the article an open access publication. Post-publication open access was funded by [funder]. Further details may be found at [MedEngine webpage link].
References
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Aurora SK, Brin MF. Chronic migraine: an update on physiology, imaging, and the mechanism of action of two available pharmacologic therapies. Headache. 2017;57(1):109–25.
Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30:599–609.
May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–64.
Bigal ME, Lipton RB. Concepts and mechanisms of migraine chronification. Headache. 2008;48:7–15.
Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep. 2012;16(1):86–92.
Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31(3):301–15.
Bigal ME, Serrano D, Reed M, et al. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–66.
Lipton RB, Serrano D, Buse DC, et al. Improving the detection of chronic migraine: development and validation of identify chronic migraine (ID-CM). Cephalalgia. 2016;36(3):203–15.
Weatherall MW. The diagnosis and treatment of chronic migraine. Ther Adv Chronic Dis. 2015;6(3):115–23.
Sun-Edelstein C, Rapoport AM. Update on the pharmacological treatment of chronic migraine. Curr Pain Headache Rep. 2016;20(1):6.
Starling AJ, Dodick DW. Best practices for patients with chronic migraine: burden, diagnosis, and management in primary care. Mayo Clin Proc. 2015;90(3):408–14.
Starling AJ, Vargas BB. A narrative review of evidence-based preventive options for chronic migraine. Curr Pain Headache Rep. 2015;19(10):49.
National Institute for Health and Care Excellence. Headaches in over 12 s: diagnosis and management. Clinical guideline [CG150]. 2015. https://www.nice.org.uk. Accessed 23 Feb 2017.
British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication-overuse headache: 3rd edition (1st revision) 2010. http://www.bash.org.uk. Accessed 23 Feb 2017.
Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine: revised report of an EFNS task force. Eur J Neurol. 2009;16:968–81.
Frampton JE. OnabotulinumtoxinA (BOTOX®): a review of its use in the prophylaxis of headaches in adults with chronic migraine. Drugs. 2012;72(6):825–45.
Health Products Regulatory Authority. BOTOX 50, 100, 200 Allergan Units, powder for solution for injection (BOTULINUM TOXIN TYPE A): IE/H/0113/001-003: IPAR. http://www.hpra.ie. Accessed 30 May 2017.
Allergan Inc. BOTOX (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use: US prescribing information. 2016. http://www.allergan.com. Accessed 11 Jan 2017.
Allergan Inc. BOTOX onabotulinumtoxinA for injection Ph. Eur: Canadian product monograph. 2014. https://allergan-web-cdn-prod.azureedge.net. Accessed 24 Feb 2017.
Whitcup SM, Turkel CC, Degryse RE, et al. Development of onabotulinumtoxinA for chronic migraine. Ann NY Acad Sci. 2014;1329:67–80.
Simpson LL. Identification of the major steps in botulinum toxin action. Ann Rev Pharmacol Toxicol. 2004;44:167–93.
Do TP, Hvedstrup J, Schytz HW. Botulinum toxin: a review of the mode of action in migraine. Acta Neurol Scand. 2018. https://doi.org/10.1111/ane.12906.
Szok D, Csati A, Vecsei L, et al. Treatment of chronic migraine with onabotulinumtoxinA: mode of action, efficacy and safety. Toxins. 2015;7(7):2659–73.
Burstein R, Zhang X, Levy D, et al. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia. 2014;34(11):853–69.
Olesen J, Burstein R, Ashina M, et al. Origin of pain in migraine: evidence for peripheral sensitization. Lancet Neurol. 2009;8(7):679–90.
Gooriah R, Ahmed F. OnabotulinumtoxinA for chronic migraine: a critical appraisal. Ther Clin Risk Manag. 2015;11:1003–13.
Cernuda-Morollón E, Ramon C, Martinez-Camblor P, et al. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain. 2015;156(5):820–4.
Luvisetto S, Gazerani P, Cianchetti C, et al. Botulinum toxin type A as a therapeutic agent against headache and related disorders. Toxins. 2015;7(9):3818–44.
Zhang X, Strassman AM, Novack V, et al. Extracranial injections of botulinum neurotoxin type A inhibit intracranial meningeal nociceptors’ responses to stimulation of TRPV1 and TRPA1 channels: are we getting closer to solving this puzzle? Cephalalgia. 2016;36(9):875–86.
Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44–61.
Bomba-Warczak E, Vevea JD, Brittain JM, et al. Interneuronal transfer and distal action of tetanus toxin and botulinum neurotoxins A and D in central neurons. Cell Rep. 2016;16(7):1974–87.
Cai BB, Francis J, Brin MF, et al. Botulinum neurotoxin type A-cleaved SNAP25 is confined to primary motor neurons and localized on the plasma membrane following intramuscular toxin injection. Neuroscience. 2017;352:155–69.
Hubbard CS, Becerra L, Smith JH, et al. Brain changes in responders vs. non-responders in chronic migraine: markers of disease reversal. Front Hum Neurosci. 2016;10:497.
Allergan Ltd. BOTOX 50 Allergan units: UK summary of product characteristics. 2015. https://www.medicines.org.uk. Accessed 11 Jan 2017.
Naumann M, Boo LM, Ackerman AH, et al. Immunogenicity of botulinum toxins. J Neural Transm. 2013;120:275–90.
Medicines and Healthcare Products Regulatory Agency. Botox (Botulinum toxin type A): PL 00426/0074-0105; PL 00426/0118-0025; PL 00426/0119-0007: UKPAR. http://www.mhra.gov.uk. Accessed 1 Mar 2017.
Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793–803.
Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–14.
Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50(6):921–36.
Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358–73.
Aurora SK, Halker R, Pozo-Rosich P, et al. The impact of onabotulinumtoxinA on severe headache days: preempt 24-week pooled analysis [abstract no. PF28 plus poster]. Headache. 2016;56(Suppl 1):27–8.
Matharu M, Halker R, Pozo-Rosich P, et al. The impact of onabotulinumtoxinA on severe headache days: preempt 24-week pooled analysis [abstract no. EHMTC-0156 plus poster]. Cephalalgia. 2016;36(Suppl 1):34–5.
Therapeutic Goods Administration. Australian public assessment report for botulinum toxin type A. http://www.tga.gov.au. Accessed 7 Mar 2017.
Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160.
Blumenfeld A, Silberstein SD, Dodick DW, et al. Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50(9):1406–18.
National Institute for Health and Care Excellence. Botulinum toxin type A for the prevention of headaches in adults with chronic migraine. Technology appraisal guidance [TA260]. 2012. https://www.nice.org.uk. Accessed 23 Mar 2017.
Silberstein SD, Blumenfeld AM, Cady RK, et al. OnabotulinumtoxinA for treatment of chronic migraine: PREEMPT 24-week pooled subgroup analysis of patients who had acute headache medication overuse at baseline. J Neurol Sci. 2013;331(1–2):48–56.
Lipton RB, Varon SF, Grosberg B, et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine. Neurology. 2011;77(15):1465–72.
Lipton RB, Rosen NL, Ailani J, et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine over one year of treatment: pooled results from the PREEMPT randomized clinical trial program. Cephalalgia. 2016;36(9):899–908.
Aurora SK, Dodick DW, Diener HC, et al. OnabotulinumtoxinA for chronic migraine: efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand. 2014;129(1):61–70.
Silberstein SD, Dodick DW, Aurora SK, et al. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86(9):996–1001.
Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of onabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13. https://doi.org/10.1186/s10194-018-0840-8.
Blumenfeld AM, Aurora SK, Laranjo K, et al. Unmet clinical needs in chronic migraine: rationale for study and design of COMPEL, an open-label, multicenter study of the long-term efficacy, safety, and tolerability of onabotulinumtoxinA for headache prophylaxis in adults with chronic migraine. BMC Neurol. 2015;15:100.
Aurora S, Stark R, Reppine A. Descriptive comparison of the efficacy and safety of onabotulinumtoxinA for headache prophylaxis in adult chronic migraine patients from two long-term, multicenter studies: COMPEL vs. PREEMPT [abstract no. PF18 plus poster]. Headache. 2015;55(Suppl 3):142.
Blumenfeld A, Stark R, Manack Adams A, et al. Efficacy and safety of onabotulinumtoxinA in an open-label study for the prophylactic treatment of chronic migraine in adult patients: COMPEL [abstract no. EHMTC-0159 plus poster]. Cephalalgia. 2016;36(Suppl 1):27–8.
Blumenfeld A, Stark R, Manack Adams A, et al. Efficacy and safety of onabotulinumtoxinA in an open-label study for the prophylactic treatment of chronic migraine in adult patients: COMPEL [abstract no. P2.178]. Neurology. 2017:88(16 Suppl 1).
Blumenfeld A, Tepper SJ, Robbins LD, et al. The effects of onabotulinumtoxinA treatment on the chronic migraine comorbidities of sleep and fatigue [abstract no. EP1078 plus poster]. Eur J Neurol. 2017;24(Suppl 1):163.
Blumenfeld AM, Tepper SJ, Robbins LD, et al. Effects of onabotulinumtoxinA treatment on the chronic migraine comorbidities of depression and anxiety [poster no. PO-02-028]. In: 18th International Headache Congress, 2017.
Young WB, Lopez JI, Rothrock JF, et al. Effects of onabotulinumtoxinA treatment on disability and quality of life in patients with chronic migraine with baseline allodynia: a COMPEL subanalysis [abstract no. PF60 plus poster]. In: American Headache Society 59th Annual Scientific Meeting, 2017.
Lopez JI, Blumenfeld AM, Young WB, et al. Effects of onabotulinumtoxinA treatment on disability and quality of life in patients with chronic migraine with baseline headache every day: a COMPEL subanalysis [abstract no. PF54 plus poster]. In: American Headache Society 59th Annual Scientific Meeting, 2017.
Young WB, Lopez JI, Rothrock JF, et al. Effects of onabotulinumtoxinA treatment on disability and quality of life in patients with chronic migraine with baseline headache every day or allodynia: a COMPEL subanalysis [poster]. In: 3rd Congress of the European Academy of Neurology, 2017.
Negro A, Curto M, Lionetto L, et al. OnabotulinumtoxinA 155 U in medication overuse headache: a two years prospective study. SpringerPlus. 2015;4:826.
Negro A, Curto M, Lionetto L, et al. A two years open-label prospective study of onabotulinumtoxinA 195 U in medication overuse headache: a real-world experience. J Headache Pain. 2015;17:1.
Khalil M, Zafar HW, Quarshie V, et al. Prospective analysis of the use of OnabotulinumtoxinA (BOTOX) in the treatment of chronic migraine; real-life data in 254 patients from Hull, UK. J Headache Pain. 2014;15:54.
Buture A, Khalil M, Nimeri R, et al. Analysis of patterns of response to onabotulinumtoxinA in chronic migraine in predicting long term outcome [abstract no. EHMTC-0343]. Cephalalgia. 2016;36(Suppl 1):17.
Buture A, Nimeri R, Khalil M, et al. Long term outcome for onabotulinumtoxinA in chronic migraine; two year follow up of 302 patients from the Hull Migraine Clinic [abstract no. EHMTC-0328]. Cephalalgia. 2016;36(Suppl 1):16–7.
Ahmed F, Zafar HW, Buture A, et al. Does analgesic overuse matter? Response to onabotulinumtoxinA in patients with chronic migraine with or without medication overuse. SpringerPlus. 2015;4:589.
Ahmed F, Buture A, Nimeri R, et al. Does medication overuse matter? Response to onabotulinumtoxina in chronic migraine (CM) with or without medication overuse; update from real-life data [abstract no. EHMTC-0340]. Cephalalgia. 2016;36(Suppl 1):12.
Ahmed F, Pascual J, Geevarghese H, et al. A 24-month, prospective, non-interventional study to describe the long term, real-life use of Botox® for the symptomatic treatment of adults with chronic migraine observed in practice (repose study): 6 months interim analysis [abstract no. LBP16 plus poster]. Headache. 2015;55(Suppl 5):264.
Pascual J, Gaul C, Davies B, et al. Real-life use of onabotulinumtoxinA for the symptomatic treatment of chronic migraine: 12-month repose study interim analysis [abstract no. EHMTC-0214 plus poster]. Cephalalgia. 2016;36(Suppl 1):39–40.
Ahmed F, Gaul C, Martelletti P, et al. Real-life use of onabotulinumtoxinA for the symptomatic treatment of chronic migraine: the REPOSE study [poster no. PO-01-066]. In: 18th International Headache Congress, 2017.
Domínquez C, Pozo-Rosich P, Torres-Ferrús M, et al. OnabotulinumtoxinA in chronic migraine: predictors of response. A prospective multicentre descriptive study. Eur J Neurol. 2017. https://doi.org/10.1111/ene.13523.
Matharu M, Pascual J, Nilsson Remahl I, et al. Utilization and safety of onabotulinumtoxinA for the prophylactic treatment of chronic migraine from an observational study in Europe. Cephalalgia. 2017;37(14):1384–97.
US National Institutes of Health. ClinicalTrials.gov identifier NCT01432379. 2016. https://clinicaltrials.gov. Accessed 17 Mar 2017.
Sarchielli P, Granella F, Prudenzano MP, et al. Italian guidelines for primary headaches: 2012 revised version. J Headache Pain. 2012;13(Suppl 2):31–70.
Scottish Medicines Consortium. Botulinum toxin A (Botox). 2017. https://www.scottishmedicines.org.uk. Accessed 23 Mar 2017.
Matharu M, Pascual J, Nilsson Remahl I, et al. Interim analysis of the real-world utilization and safety of onabotulinumtoxinA for the prophylactic treatment of chronic migraine in an observational study in the European Union [abstract no. PO051 plus poster]. Cephalalgia. 2015;35(Suppl 6):39.
Matharu M, Pascual J, Nilsson Remahl I, et al. Real-world treatment utilization and safety of onabotulinumtoxinA for chronic migraine from an observational study in the European Union [abstract EHMTC-0143 plus poster]. Cephalalgia. 2016;36(Suppl 1):27.
Sarchielli P, Romoli M, Corbelli I, et al. Stopping onabotulinum treatment after the first two cycles might not be justified: results of a real-life monocentric prospective study in chronic migraine. Front Neurol. 2017;8:655.
Tassorelli C, Tedeschi G, Sarchielli P, et al. Optimizing the long-term management of chronic migraine with onabotulinumtoxinA in real life. Expert Rev Neurother. 2018;18(2):167–76.
Cernuda-Morollón E, Martinez-Camblor P, Ramon C, et al. CGRP and VIP levels as predictors of efficacy of onabotulinumtoxin type A in chronic migraine. Headache. 2014;54(6):987–95.
Moreno R, Ruiz M, Cernuda-Morollón E, et al. Calca gene polymorphism influences therapeutic response to onabotulinumtoxin A in female chronic migraine patients [abstract no. EHMTC-0368]. Cephalalgia. 2016;36(Suppl 1):80.
Moreno R, Ruiz M, Cernuda-Morollón E, et al. Pharmacogenetics in chronic migraine: role of CALCA and TRPV1 genes in therapeutic response to onabotulinumtoxin A [abstract no. O1110]. Eur J Neurol. 2017;24(Suppl 1):25.
Khalil M, Buture A, Nimeri R, et al. OnabotulinumtoxinA in chronic migraine; predicting response to treatment based on headache days at baseline [abstract no. EHMTC-0332]. Cephalalgia. 2016;36(Suppl 1):118–9.
Ruggeri M. The cost effectiveness of Botox in Italian patients with chronic migraine. Neurol Sci. 2014;35(Suppl 1):45–7.
Batty AJ, Hansen RN, Bloudek LM, et al. The cost-effectiveness of onabotulinumtoxinA for the prophylaxis of headache in adults with chronic migraine in the UK. J Med Econ. 2013;16(7):877–87.
Kollewe K, Oberling M, Kiszka M, et al. OnabotulinumtoxinA for the symptomatic treatment of chronic migraine: REPOSE study 12-month interim analysis of health-economic indicators in German patients [abstract no. LB 899]. In: 89th Congress of the German Society of Neurology, 2016.
Acknowledgements
During the peer review process, the manufacturer of onabotulinumtoxinA was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
James Frampton is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
As a consultant and/or advisory panel member, Stephen Silberstein receives, or has received, honoraria from: Alder Biopharmaceuticals; Allergan, Inc.; Amgen; Avanir Pharmaceuticals, Inc.; Curelator, Inc.; Depomed; Dr. Reddy’s Laboratories; eNeura Inc.; electroCore Medical, LLC; INSYS Therapeutics; Labrys Biologics; Lilly USA, LLC; Medscape, LLC; Medtronic, Inc.; Neuralieve; NINDS; Pfizer, Inc.; Supernus Pharmaceuticals, Inc.; Teva Pharmaceuticals; Theranica; and Trigemina, Inc.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/457AF0606D3C2B1C.
Additional information
The manuscript was reviewed by: A. Blumenfeld, The Neurology Center, Carlsbad, CA, USA; H. C. Diener, Medical Faculty and Department of Neurology, University Duisburg-Essen, Essen, Germany; F. Freitag, Department of Neurology, The Medical College of Wisconsin, Milwaukee, WI, USA; K. Kollewe, Department of Neurology, Movement Disorder Section, Hannover Medical School, Hannover, Germany; W. Schulte-Mattler, Department of Neurology, University Hospital Regensburg, Regensburg, Germany; R. J. Stark, Van Cleef Department of Neuroscience, Monash University, Melbourne, Australia.
The original version of this article was revised due to a retrospective Open Access request.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Frampton, J.E., Silberstein, S. OnabotulinumtoxinA: A Review in the Prevention of Chronic Migraine. Drugs 78, 589–600 (2018). https://doi.org/10.1007/s40265-018-0894-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0894-6