Abstract
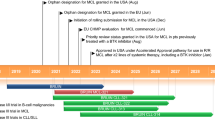
Bayer are developing copanlisib (Aliqopa™)—a pan-class I phosphoinositide 3-kinase (PI3K) inhibitor—as a treatment for various haematological and solid malignancies. The US FDA has granted copanlisib accelerated approval for the treatment of adults with relapsed follicular lymphoma who have received at least two prior systemic therapies based on the results of a phase II trial. Phase III trials are underway evaluating copanlisib as treatment for relapsed/refractory diffuse large B-cell lymphoma and in combination with rituximab or rituximab-based chemotherapy or standard immunochemotherapy in patients with relapsed indolent B-cell non-Hodgkin’s lymphoma. Phase I/II studies are underway in relapsed or refractory peripheral T-cell or NK/T-cell lymphoma, advanced cholangiocarcinoma, hormone receptor-positive HER2-negative stage I-IV breast cancer, HER2-positive breast cancer and recurrent and/or metastatic head and neck squamous cell carcinomas harbouring a PI3KCA mutation/amplification and/or a PTEN loss. This article summarizes the milestones in the development of copanlisib leading to this first approval for relapsed follicular lymphoma.
Similar content being viewed by others
References
Liu N, Rowley BR, Bull CO, et al. BAY 80-6946 is a highly selective intravenous pI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12(11):2319–30.
Bayer. FDA grants Bayer priority review for investigational compound copanlisib in follicular lymphoma [media release]. 17 May 2017.
FDA. Aliqopa (Copanlisib) prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209936s000lbl.pdf. Accessed 10 Oct 2017.
Glauer J, Pletz N, Schon M, et al. A novel selective small-molecule PI3K inhibitor is effective against human multiple myeloma in vitro and in vivo. Blood Cancer J. 2013;3:e141.
Paul J, Wengner AM, Petrova E, et al. Copanlisib attenuates both BCR-dependent and BCR-independent activation of NFKB in DLBCL cells [abstract no. 268]. Hematol Oncol. 2015;33(Suppl 1):235.
Zoellner A, Arnd J, Hutter G, et al. Efficacy of the pan PI3K-inhibitor copanlisib compared to selective PI3K-a,-b,-delta inhibitors in mantle cell lymphoma (MCL) [abstract no. E1375]. Haematologica. 2015;100(Suppl 1):551.
Patnaik A, Appleman LJ, Tolcher AW, et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann Oncol. 2016;27:1928–40.
Gerisch M, Schwarz T, Lang D, et al. Pharmacokinetics of intravenous pan-class I phosphatidylinositol 3-kinase (PI3K) inhibitor [14C]copanlisib (BAY 80-6946) in a mass balance study in healthy male volunteers. Cancer Chemother Pharmacol. 2017;80(3):535–44.
Reif S, Ahsman M, Jentsch G, et al. Use of a population pharmacokinetic approach and time-to-event analysis to support the clinical recommendation of a flat dosing of copanlisib in cancer patients [abstract no. PI-093]. Clin Pharmacol Ther. 2016;99(Suppl 1):S55.
Gerecitano J, Santoro A, Leppa S, et al. Safety run-in of copanlisib in combination with rituximab plus bendamustine in patients with relapsed indolent non-Hodgkin’s lymphoma [abstract no. 477]. Hematol Oncol. 2017;35(Suppl 2):408–10.
Dreyling M, Morschhauser F, Bouabdallah K, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169–78.
Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017. https://doi.org/10.1200/jco.2017.75.4648.
Lenz G, Hawkes E, Verhoef G, et al. Clinical outcomes and molecular characterization from a phase II study of copanlisib in patients with relapsed or refractory diffuse large B-cell lymphoma [abstract no. 57]. Hematol Oncol. 2017;35(Suppl 2):68–9.
Ramanathan RK, Von Hoff DD, Eskens F, et al. A phase 1b trial of PI3K inhibitor copanlisib (BAY 80-6946) combined with the allosteric-MEK inhibitor refametinib (BAY 86-9766) in patients with advanced cancer [abstract no. 2588]. J Clin Oncol Conf. 2014;32(Suppl 1).
Kim RD, Alberts SR, Renshaw FG, et al. Phase 1 dose escalation study of copanlisib (BAY 80-6946) in combination with gemcitabine or gemcitabine-cisplatin in advanced cancer patients [abstract no. 2610]. J Clin Oncol Conf. 2014;32(Suppl 1).
Doi T, Fuse N, Yoshino T, et al. A phase I study of intravenous PI3K inhibitor copanlisib in Japanese patients with advanced or refractory solid tumors. Cancer Chemother Pharmacol. 2017;79:89–98.
Nowakowski GS, Gorbatchevsky I, Hiemeyer F, et al. A randomized, double-blind phase III study of phosphatidylinositol 3 kinase alpha/delta inhibitor copanlisib versus placebo in patients with rituximab refractory indolent non Hodgkin’s lymphoma (iNHL): CHRONOS2 [abstract no. CT084]. Cancer Res. 2016;76(14 Suppl).
Gerecitano JF, Zinzani PL, Zheng HX, et al. Phase III randomized, double-blind, controlled studies of the PI3K inhibitor copanlisib in combination with rituximab or rituximab-based chemotherapy in subjects with relapsed indolent B-cell non-Hodgkin’s lymphoma (iNHL): CHRONOS-3 and CHRONOS-4 [abstract no. CT085]. Cancer Res. 2016;76(14 Suppl).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham, a contracted employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/F6CCF0601D893768.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Copanlisib: First Global Approval. Drugs 77, 2057–2062 (2017). https://doi.org/10.1007/s40265-017-0838-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0838-6