Abstract
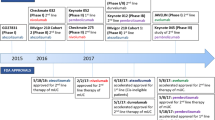
Cytotoxic chemotherapy has been the only systemic treatment of locally advanced and metastatic urothelial carcinoma for decades. Long-term survival remains stagnant around 12–14 months for patients with advanced disease who have progressed on or recurred after receiving first-line platinum-based chemotherapy. Improving clinical outcomes for patients with urothelial carcinoma in all disease settings requires the development of novel treatments, especially for patients who failed on first-line chemotherapy. Since the discovery of intravesical Bacillus-Calmette Guerin (BCG) in the 1970s for non-muscle invasive disease, there have not been any major breakthrough drugs that exploit the immune-sensitivity of bladder cancer until recently. Immune-checkpoint inhibitors targeting the programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways have shown significant anti-tumor activity, tolerable safety profiles and durable, long-term responses in clinical trials. Atezolizumab, avelumab, durvalumab, nivolumab and pembrolizumab are promising PD-1/PD-L1 blockade drugs under investigation that will redefine the standard of care for bladder cancer. CTLA-4 inhibitors are also under investigation in this setting. Atezolizumab, approved in May 2016, and nivolumab, approved in February 2017, are the first Food and Drug Administration (FDA)-approved immune-checkpoint inhibitors in bladder cancer for platinum-pretreated patients based on phase II data. On March 16, 2017, results from the phase III trial KEYNOTE-045 demonstrated that survival was significantly longer in patients treated with pembrolizumab when compared with the standard second-line chemotherapy. Research into biomarkers such as PD-L1 expression, messenger RNA subtype, mutational and neoantigen load and gene signature expression will be crucial to determining why some patients respond to immunotherapy and others do not. This review article describes the advances in immunotherapy since the development of BCG, presents results from clinical trials investigating immune-checkpoint inhibitors and discusses biomarkers and prognostic factors associated with response to these new drugs.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387.
Bladder cancer risk factors. http://www.cancer.org/cancer/bladdercancer/detailedguide/bladder-cancer-risk-factors. Published May 23, 2016. Accessed December 18, 2016.
NCI. Surveillance, Epidemiology, and End Results Program. Cancer of the Urinary Bladder—SEER Stat Fact Sheets. 2016. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed December 18, 2016.
Dueker DC, Stern MC, Conti DV, Pike MC, Cortessis VK. Abstract 3622: further observation of increasing strength of the smoking-bladder cancer association: the Los Angeles Bladder Cancer Study. Cancer Res. 2013;73(8 Supplement):3622. doi:10.1158/1538-7445.am2013-3622.
Survival rates for bladder cancer. American Cancer Society. 2016. http://www.cancer.org/cancer/bladdercancer/detailedguide/bladder-cancer-survival-rates. Accessed December 18, 2016.
Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–5. doi:10.1200/jco.2009.25.4599.
Galsky MD, Hahn NM, Rosenberg J, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet. 2011;12(3):211–4. doi:10.1016/s1470-2045(10)70275-8.
Yafi FA, North S, Kassouf W. First- and second-line therapy for metastatic urothelial carcinoma of the bladder. Curr Oncol. 2011. doi:10.3747/co.v18i1.695.
Bajorin DF, Dodd PM, Mazumdar M. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173–81. doi:10.1200/jco.1999.17.10.3173.
Sylvester RJ. Bacillus Calmette–Guérin treatment of non-muscle invasive bladder cancer. Int J Urol. 2010;18(2):113–20. doi:10.1111/j.1442-2042.2010.02678.x.
Zbar B, Rapp HJ. Immunotherapy of guinea pig cancer with BCG. Cancer. 1974;34(S8):1532–40. doi:10.1002/1097-0142(197410)34:8.
Morales A, Eidinger D, Bruce A. Intracavitary bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J Urol. 2002;167(2):891–4. doi:10.1016/s0022-5347(02)80294-4. (reprint of J Urol. 1976;116:180–183)
Herr HW, Morales A. History of bacillus Calmette–Guerin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53–6. doi:10.1016/j.juro.2007.08.122.
Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette–Guerin immunotherapy for recurrent Ta, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163(4):1124–9. doi:10.1016/s0022-5347(05)67707-5.
Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette–Guérin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325(17):1205–9. doi:10.1056/nejm199110243251703.
TICE BCG. 2016. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm134033.htm. Accessed December 18, 2016.
Bladder Cancer. Cancer Research Institute. http://www.cancerresearch.org/cancer-immunotherapy/impacting-all-cancers/bladder-cancer. Published 2016. Accessed November 30, 2016.
Poschke I, Mougiakakos D, Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother. 2011;60(8):1161–71.
Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–66. doi:10.4049/jimmunol.170.3.1257.
Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119–25. doi:10.1200/jco.2016.67.9761.
Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8 T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–5. doi:10.1073/pnas.0611533104.
Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106.
Sundararajan S, Vogelzang NJ. Anti-PD-1 and PD-L1 therapy for bladder cancer: what is on the horizon? Future Oncol. 2015;11(16):2299–306. doi:10.2217/fon.15.162.
Dai S-X, Wu G, Zou Y, et al. Balance of CD8+ CD28+/CD8+ CD28− T lymphocytes is vital for patients with ulcerative colitis. Dig Dis Sci. 2012;58(1):88–96. doi:10.1007/s10620-012-2327-9.
Ménager-Marcq I, Pomié C, Romagnoli P, Meerwijk JPV. CD8+ CD28− regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterol. 2006;131(6):1775–85. doi:10.1053/j.gastro.2006.09.008.
Merwe PAVD, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185(3):393–404. doi:10.1084/jem.185.3.393.
Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11(1):191–212. doi:10.1146/annurev.iy.11.040193.001203.
Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Expl Med. 2009;206(8):1717–25. doi:10.1084/jem.20082492.
van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2001;21:659–84. doi:10.1146/annurev.immunol.21.120601.141036.
Carreno BM, Bennett F, Chau TA, et al. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165(3):1352–6. doi:10.4049/jimmunol.165.3.1352.
Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. doi:10.1038/nature13904.
FDA approves Roche’s cancer immunotherapy TECENTRIQ (atezolizumab) for people with a specific type of metastatic lung cancer. M2 Presswire. http://search.proquest.com.ezproxy.library.tufts.edu/docview/1830015330/abstract/5E40FDE609A84CF7PQ/1?accountid=14434. Published October 19, 2016. Accessed December 18, 2016.
FDA approves new immunotherapy drug for bladder cancer. National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2016/fda-atezolizumab-bladder. Published June 7, 2016. Accessed December 18, 2016.
Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi:10.1016/s0140-6736(16)32455-2.
Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–20. doi:10.1016/s0140-6736(16)00561-4.
Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590–8. doi:10.1016/s1470-2045(16)30496-x.
Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–22. doi:10.1016/s1470-2045(17)30065-7.
O’Donnell PH, Plimack ER, Bellmunt J, et al. Pembrolizumab (Pembro; MK-3475) for advanced urothelial cancer: results of a phase IB study. [supplement no. 7; abstract no. 296]. In: ASCO Genitourinary Cancers Symposium; 2015.
Plimack ER, Bellmunt J, Gupta S, et al. Pembrolizumab (MK-3475) for advanced urothelial cancer: updated results and biomarker analysis from KEYNOTE-012. [supplement; abstract no. 4502]. In: ASCO Annual Meeting; 2015.
Bellmunt J, Wit RD, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–26. doi:10.1056/nejmoa1613683.
Durvalumab granted Breakthrough Therapy designation by US FDA for treatment of patients with PD-L1 positive urothelial bladder cancer. AstraZeneca.com. https://www.astrazeneca.com/media-centre/press-releases/2016/Durvalumab-granted-Breakthrough-Therapy-designation-by-US-FDA-for-treatment-of-patients-with-PD-L1-positive-urothelial-bladder-cancer-17022016.html. Published February 17, 2016. Accessed December 18, 2016.
Powles T, O’Donnell PH, Massard C, et al. Updated efficacy and tolerability of durvalumab in locally advanced or metastatic urothelial carcinoma [abstract no. 286]. In: ASCO Genitourinary Cancers Symposium, 2017.
Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017. doi:10.1200/jco.2016.71.6795.
von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–77. doi:10.1200/JCO.2000.18.17.3068.
Sternberg CN, Mulder PHD, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor vs. classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol. 2001;19(10):2638–46. doi:10.1200/jco.2001.19.10.2638.
Bellmunt J, Maase HVD, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC intergroup study 30987. J Clin Onc. 2012;30(10):1107–13. doi:10.1200/jco.2011.38.6979.
Santis MD, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC Study 30986. J Clin Oncol. 2011;30(2):191–9. doi:10.1200/jco.2011.37.3571.
Santis MD, Wiechno PJ, Bellmunt J, et al. Vinflunine–gemcitabine vs. vinflunine–carboplatin as first-line chemotherapy in cisplatin-unfit patients with advanced urothelial carcinoma: results of an international randomized phase II trial (JASINT1). Ann Oncol. 2015;27(3):449–54. doi:10.1093/annonc/mdv609.
Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454–61. doi:10.1200/jco.2008.20.5534.
Balar AV, Castellano DE, O’Donnell PH, et al. Pembrolizumab as first-line therapy in cisplatin-ineligible advanced urothelial cancer: results from the total KEYNOTE-052 study population. [abstract no. 284]. In: ASCO Genitourinary Cancers Symposium, 2017.
Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26(4):812–7. doi:10.1093/annonc/mdv009.
Weinstein JN, Akbani R, Broom BM, et al. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22. doi:10.1038/nature12965.
Sweis RF, Spranger S, Bao R, et al. Molecular drivers of the non-T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol Res. 2016;4(7):563–8. doi:10.1158/2326-6066.cir-15-0274.
Rosenberg JE, Petrylak DP, Van Der Heijden MS, et al. PD-L1 expression, Cancer Genome Atlas (TCGA) subtype, and mutational load as independent predictors of response to atezolizumab (atezo) in metastatic urothelial carcinoma (mUC, IMvigor210). [supplement; abstract no. 104]. In: ASCO Annual Meeting; 2016.
Campesato LF, Barroso-Sousa R, Jimenez L, et al. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget. 2015;6(33):34221–7. doi:10.18632/oncotarget.5950.
Ribas A, Robert C, Hodi FS, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. [supplement; abstract no. 3001]. In: ASCO Annual Meeting; 2015.
Balar A, Bellmunt J, O’Donnell PH, et al. Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: preliminary results from the phase 2 KEYNOTE-052 study. In: ESMO Annual Meeting; 2016.
Sharma P, Callahan MK, Calvo E et al. Efficacy and safety of nivolumab plus ipilimumab in previously treated metastatic urothelial carcinoma: first results from the phase I/II Check Mate 032 study. In: 2016 SITC Annual Meeting; November 9–13, 2016.
FDA approves new immunotherapy drug for bladder cancer. Cancer.gov. https://www.cancer.gov/news-events/cancer-currents-blog/2016/fda-atezolizumab-bladder. Published June 7 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
Matthew S. Farina and Kevin T. Lundgren declare that they have no conflict of interest. Dr. Joaquim Bellmunt reports personal fees from Merck & Co., Inc., Genentech, AstraZeneca, Bristol-Myers Squibb, EMD Serono, and Pfizer outside the submitted work.
Rights and permissions
About this article
Cite this article
Farina, M.S., Lundgren, K.T. & Bellmunt, J. Immunotherapy in Urothelial Cancer: Recent Results and Future Perspectives. Drugs 77, 1077–1089 (2017). https://doi.org/10.1007/s40265-017-0748-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0748-7