Abstract
The role of aberrant hepatocyte growth factor receptor (c-MET, also known as tyrosine-protein kinase MET)/hepatocyte growth factor (HGF) signaling in cancer progression and invasion has been extensively studied. c-MET inhibitors have shown promising pre-clinical and early phase clinical trial anti-tumor activity in several tumor types, although results of most phase III trials with these agents have been negative. To date, two small molecule c-MET inhibitors, cabozantinib and crizotinib, have been approved by regulatory authorities for the treatment of selected cancer types, but several novel c-MET inhibitors (either monoclonal antibodies or small molecule c-MET tyrosine kinase inhibitors) and treatment combinations are currently under study in different settings. Here we provide an overview of the mechanism of action and rationale of c-MET inhibition in cancer, the efficacy of approved agents, and novel promising c-MET-inhibitors and novel targeted combination strategies under development in different cancer types, with a focus on the safety profile and tolerability of these compounds.
Similar content being viewed by others
Approved hepatocyte growth factor receptor (c-MET) inhibitors cabozantinib and crizotinib show a manageable safety profile and good tolerability; nevertheless, grade 3 or 4 and fatal or life-threatening adverse events have been observed in a small percentage of cases and have to be carefully monitored. |
Hepatic function and drug–drug interactions must be considered when administering these agents, as they are primary metabolized by cytochrome P450 3A. |
Ongoing studies will further assess c-MET inhibitors tolerability and safety; however, improvements in biomarker-driven patient selection are warranted to optimize outcomes and toxicity exposure. |
1 Introduction
Receptor tyrosine kinases (RTKs) are cellular surface receptors involved in transduction pathways that modulate essential cellular processes such as metabolism, differentiation, proliferation, migration, and cell cycle [1]. Disruption and aberrant activation of RTKs have been associated with oncogenesis and tumor progression, identifying these molecules as key pharmacological targets in cancer treatment. The hepatocyte growth factor receptor (c-MET, also known as tyrosine-protein kinase MET) pathway plays a central role in tissue patterning during early embryogenesis, wound healing, and post-injury tissue regeneration [2,3,4]. Aberrant MET expression is widely observed in several cancer types and the abnormal activation of c-MET signaling has been implicated in tumor development and metastatic progression of various solid malignancies [5, 6]. Furthermore, high c-MET expression is associated with poor prognosis and resistance to targeted treatment in cancer patients [7,8,9,10]. Based on this evidence, the c-MET axis has been exploited as an intriguing therapeutic target for drug development in different types of tumor.
Several small-molecule c-MET inhibitors have been developed over the last decade and have entered clinical evaluation either as monotherapy or in combination with other agents. To date, cabozantinib (Cabometyx® and Cometriq®, Exelixis Inc., San Francisco, USA) and crizotinib (Xalkori®, Pfizer, New York, USA) are the only c-MET inhibitors that have received US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) approval in selected cancer types (Table 1) [11,12,13,14,15,16]. Meanwhile, norcantharidin (NCTD), a dual inhibitor for c-MET and epidermal growth factor receptor (EGFR), has been approved by local regulatory authorities for liver, esophageal, and gastric cancer treatment in China since 1996 [17]. Despite pre-clinical evidence of anti-cancer activity and initial promising results in early phase trials, most phase II and III trials failed to demonstrate clinical efficacy for most c-MET inhibitors in several tumor types [18]. The main issues behind the failure of these trials have been related to patient selection, i.e., the identification of effective biomarkers to select those patients who are likely to derive most benefit from targeted c-MET inhibition, and primary resistance mechanisms to monotherapy treatment. Hence, current efforts are directed towards the development of novel agents, implementation of predictive biomarkers and design of combination strategies to improve patient outcomes. In this scenario, a deep understanding of c-MET inhibitors’ toxicity profiles and the potential for overlapping toxicities in combination strategies is crucial.
In this review, we will present an overview of approved and novel promising c-MET-inhibitors, with a focus on their safety profile and tolerability.
2 Mechanism of Action
C-MET, located on chromosome 7q21-31, is a proto-oncogene encoding the RTK protein c-MET, whose only natural ligand is the hepatocyte growth factor (HGF), a paracrine signaling molecule secreted by mesenchymal cells. Binding of HGF results in c-MET dimerization and trans-phosphorylation of the tyrosine residues in the intracellular kinase domain, followed by further phosphorylation of tyrosine residues within the C-terminal multifunctional docking site (also known as MET binding domain). This autophosphorylation leads to the recruitment of signaling effectors like growth factor receptor-bound protein 2 (GRB2) and Grb2-associated adaptor protein 1 (GAB1), which in turn activates downstream signaling pathways such as Ras/Raf/MEK/MAPK, Ras/PAK, SRC, FAK, JAK/STAT, Wnt/β-catenin and PI3K/Akt/mTOR pathways (Fig. 1) [6, 19]. Catalytic activation of downstream signaling transduction regulates several cellular processes including cell cycle, survival, proliferation, motility, invasion, anti-apoptotic effects, epithelial-to-mesenchymal transition (EMT), and angiogenesis [6, 19]. Aberrant c-MET regulation has been observed in different tumor types, predominantly in gastrointestinal (GI) cancer, hepatocellular carcinoma (HCC), and non-small cell lung cancer (NSCLC). Abnormal activation can occur through c-MET overexpression, genomic rearrangements (translocations, amplifications, and mutations, especially in the exon 14, i.e., an exon 14 skip resulting in delayed degradation and prolonged signaling), alternative splicing, or autocrine or paracrine ligand stimulation [20, 21]. Additionally, the HGF/c-MET signaling pathway exhibits significant cross-talk with other RTK-mediated signaling pathways, such as RON (recepteur d’origine nantais) and the EGFR pathway, promoting tumorigenesis and targeted treatment resistance [6].
Main signaling pathways activated through the hepatocyte growth factor (HGF)/hepatocyte growth factor receptor (c-MET) axis and representative HGF/c-MET signaling inhibition strategies. Binding of HGF results in c-MET dimerization and phosphorylation of the tyrosine residues in the intracellular kinase domain. This autophosphorylation leads to the recruitment of signaling effectors like growth factor receptor-bound protein 2 (GRB2) and Grb2-associated adaptor protein 1 (GAB1), which in turn activate downstream signaling pathways such as signal transducer and activator of transcription (STAT), son of sevenless (SOS), proto-oncogene tyrosine-protein kinase SRC (c-SRC), phosphatidylinositol 3-kinase (PI3K), SRC homology domain c-terminal adaptor homolog (SHC), SRC homology protein tyrosine phosphatase 2 (SHP2), and phospholipase Cγ (PLCγ). Further activation of signaling cascades occurs through phosphorylation of SOS/rat sarcoma oncogene homolog (Ras)/RAF proto-oncogene serine/threonine-protein kinase (Raf)/mitogen-activated protein kinases MEK and MAPK, SOS/Ras/Ras-related C3 botulinum toxin substrate (Rac)/GTP-binding protein RHO (Rho)/p21-activated kinase (PAK), SOS/focal adhesion kinase (FAK), phosphatidylinositol 3-kinase (PI3K)/FAK, and PI3K/protein kinase B alpha (Akt)/mammalian target of rapamycin (mTOR)/nuclear factor-κB (NF-κB), among others. The activation of these signaling pathways leads to increased cell survival and proliferation, cell motility and invasion, and epithelial-to-mesenchymal transition (EMT). The different therapeutic approaches developed to target the HGF/c-MET axis have been represented. HGF/c-MET inhibition has been approached through two mechanisms of action: monoclonal antibodies (mAbs), anti-HGF and anti-c-MET, and small-molecule inhibitors of the c-MET activity, further divided into adenosine triphosphate (ATP)-competitive inhibitors (selective class I and non-selective class II for c‑MET) and non-ATP allosteric competitors (class III)
Inhibition of the HGF/c-MET axis has been approached through two main different mechanisms of action. A first approach has been the development of monoclonal antibodies (mAbs) that impair HGF/c-MET interaction and/or c-MET dimerization. A second approach is through small molecule c-MET kinase inhibitors, which target activation sites of the cytoplasmic domain of the receptor, blocking its phosphorylation and downstream signaling (Fig. 1) [21, 22]. Anti-MET mAbs are further classified by their target, based on whether they bind to the ligand HGF or to the receptor c-MET [21, 22]. Small molecule c-MET kinase inhibitors are further divided into three classes. Both class I and II are known as adenosine triphosphate (ATP)-competitive, as they serve as analogs of ATP, the substrate for phosphorylation of c-MET tyrosine kinase. The difference between these classes is based on how they bind to c-MET. Class I inhibitors are U-shaped and specifically bind to the ATP-binding site of c-MET (often via hydrogen bonding with the residue Met1160), thus showing higher selectivity than class II inhibitors [21, 23]. Class II inhibitors are multi-targeted kinase inhibitors that usually adopt an extended conformation involving additional residues, which often leads to compounds with high molecular weights and high lipophilicities [21, 23]. Finally, class III c-MET inhibitors are atypical non–ATP-competitive small molecule inhibitors. Recent advances in chemistry development of small molecule c-MET kinase inhibitors are extensively reviewed elsewhere [20].
3 Currently FDA- and EMA-Approved Agents: Efficacy Results from Main Clinical Trials
3.1 Crizotinib
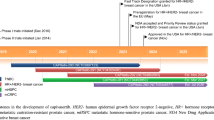
Crizotinib (Xalkori®, Pfizer) is a class II multi-target oral tyrosine kinase inhibitor (TKI) of c-MET, anaplastic lymphoma kinase (ALK), and ROS proto-oncogene 1 (ROS1) [24]. On August 26, 2011, crizotinib became the first ever targeted agent approved by the FDA for the treatment of locally advanced or metastatic ALK fusion-positive NSCLC (representing about 3–5% of all NSCLC) as detected by an FDA-approved test. This indication was followed in 2016 by the approval for treatment of ROS1-positive metastatic NSCLC (comprising about 1% of this tumor type), and more recently, in May 2018, by a breakthrough therapy designation for the treatment of patients with metastatic NSCLC with MET exon 14 alterations (about 3–4% of all NSCLC) progressing after a previous platinum-based chemotherapy. The FDA also granted in May 2018 the designation to crizotinib for use in patients with relapsed/refractory ALK-positive anaplastic large cell lymphoma (ALCL). Crizotinib is currently approved by EMA for the treatment of adults with ALK-positive advanced NSCLC and ROS1-positive NSCLC. The recommended dosage is 250-mg oral capsules twice daily.
First FDA approval was based on preliminary results of two multicenter, single-arm studies: the phase II PROFILE 1005 trial and a part 2 expansion cohort of the phase I PROFILE 1001. At time of final data presentation, these studies had enrolled 1215 patients with locally advanced or metastatic ALK-positive NSCLC. In PROFILE 1005 (N = 1066), the overall objective response rate (ORR) (primary endpoint) was 52% [25, 26], while in PROFILE 1001 (N = 149), the ORR was 60.8% [27]. The confirmatory randomized, open-label, phase III study PROFILE 1007 compared the efficacy and safety of crizotinib to standard of care, either pemetrexed or docetaxel, in the second-line treatment setting [28]. A total of 347 patients were enrolled. Median progression-free survival (mPFS) was significantly longer with crizotinib compared to chemotherapy [7.7 vs 3.0 months, hazard ratio (HR) 0.49; 95% confidence interval (CI) 0.37–0.64, P < 0.0001]. Response rates to crizotinib, pemetrexed and docetaxel were 65.7, 29.3 and 6.9%, respectively. The activity of crizotinib in previously untreated advanced ALK-positive NSCLC was confirmed by the phase III PROFILE 1014 trial comparing safety and efficacy of first-line crizotinib to either cisplatin or carboplatin plus pemetrexed in 343 patients [29, 30]. The study met its primary endpoint, progression-free survival (PFS), favoring the crizotinib arm (mPFS 10.9 vs 7 months, HR 0.45, P > 0.001). Four-year overall survival (OS) was 56.6% and 49.1% with crizotinib and chemotherapy, respectively. Of note, treatment with crizotinib was associated with a greater improvement in patient-reported quality-of-life outcomes and better symptom control.
The 2016 approval for the treatment of ROS1-positive metastatic NSCLC was based on a multicenter, prospective, single-arm trial enrolling 50 patients whose tumors were determined to be ROS1-positive by fluorescence in situ hybridization (FISH) (96%) or reverse transcription polymerase chain reaction (RT-PCR) (4%) assays. The ORR was 72% (95% CI 58–84), with an mPFS of 19.2 months [31].
The 2018 breakthrough designation for c-MET-positive NSCLC was based on results of an expansion cohort from the phase I PROFILE 1001 study, including 21 patients with c-MET exon 14–altered NSCLC. Eight cases of partial response (PR) (44%) and nine of stable disease (SD) (50%) were observed among 18 evaluable patients [32].
Finally, the breakthrough designation for the treatment of ALCL was based on two trials. The first study was the multicenter, single-arm, open-label phase Ib A8081013 study, which evaluated crizotinib in 44 adult patients with ALK-positive advanced malignancies other than NSCLC [33]. This trial included 18 patients with lymphoma, nine with inflammatory myofibroblastic tumors (IMTs), and 17 with other tumors. Among patients with lymphoma, eight complete response (CR) and one PR were observed, with a 2-year PFS rate of 63%. The second trial, ADVL0912, investigated crizotinib in 40 pediatric patients comprising 26 relapsed/refractory ALK-positive ALCL and 14 metastatic or inoperable ALK-positive IMT [34]. Patients with ALCL received crizotinib at two different adjusted doses: 165 mg/m2 (N = 6) or 280 mg/m2 (N = 20). The ORRs were 83% (five of six CR) and 90% (16 CR and two PR out of 20) in the two treatment arms, respectively.
Crizotinib is currently under clinical evaluation in several other tumor types, including breast, prostate, glioblastoma, uveal melanoma, and MET-amplified gastric adenocarcinoma. A summary of the main trials of interest across different tumors and novel treatment combinations with other drugs or targeted agents can be found in Table 2 [35,36,37,38,39,40,41,42].
3.2 Cabozantinib
Cabozantinib is an oral TKI targeting c-MET, vascular endothelial growth factor receptor (VEGFR)-1, VEGFR2 and VEGFR3, REarranged during Transfection (RET), tyrosine-protein kinase receptor UFO (AXL), KIT, tyrosine receptor kinase B (TRKB), fms like tyrosine kinase 3 (FLT-3), and TIE-2 [43], which showed preclinical and clinical activity in several types of tumors, including thyroid cancer [44, 45], renal cell carcinoma (RCC) [46], prostate [47], and NSCLC [48, 49]. To date, cabozantinib has been approved by the FDA for two indications: advanced RCC (Cabometyx®, Exelixis, Inc.) and progressive, locally advance and metastatic medullary thyroid cancer (MTC) (Cometriq®, Exelixis, Inc.). Of note, Cometriq® and Cabometyx® have different formulations and are not interchangeable. Cabometyx® is currently approved by EMA for the treatment of advanced RCC in treatment-naïve adults with intermediate- or poor-risk tumors or following prior anti-VEGF treatment. Cometriq® is indicated for the treatment of adult patients with progressive, unresectable locally advanced or metastatic MTC.
Cometriq® is supplied as a capsule for oral administration. The recommended daily dose is 140 mg. The 2012 FDA approval of Cometriq® was based on an international, multicenter, randomized, double-blind, controlled trial in 330 subjects with metastatic MTC [50]. A statistically significant prolongation in PFS was demonstrated favoring the cabozantinib arm (P < 0.0001), with a mPFS of 11.2 and 4.0 months in the Cometriq® and placebo arms, respectively. PRs were observed only among subjects in the Cometriq® arm (27% vs 0%; P < 0.0001). The median duration of objective responses was 14.7 months for subjects treated with Cometriq®.
On the basis of its unique targeting profile, including RET and VEGFR in addition to c-MET, and promising activity demonstrated in a phase I study [51], cabozantinib has been tested in differentiated thyroid cancer (DTC) patients with radioiodine (RAI)-refractory disease progressing on prior VEGFR-targeted treatment [52]. In this setting, cabozantinib demonstrated clinically significant and durable objective response activity with 40% PR and 52% SD out of the 25 patients enrolled (8% non-evaluable disease). Median PFS and OS were 12.7 and 34.7 months, respectively.
In 2016, the FDA approved Cabometyx® for the treatment of advanced RCC in patients who have received prior anti-angiogenic therapy, based on results of the phase III METEOR trial [53]. Later, in December 2017, the FDA granted first-line approval to Cabometyx® for the treatment of patients with advanced RCC, based on results of the CABOSUN trial [54]. In the METEOR trial [53, 55], 658 patients with advanced RCC who had received at least one prior VEGFR TKI were randomized to Cabometyx® (60 mg daily) or everolimus. Cabozantinib significantly improved PFS (7.4 and 3.8 months, HR 0.58, 95% CI 0.45–0.74; P < 0.0001), OS (21.0 vs 17.1 months, HR 0.70, 95% CI 0.58–0.85; P = 0.0002) and ORR (17% vs 3%) compared to everolimus [56]. The randomized, open-label, phase II CABOSUN trial demonstrated an improvement in PFS from first-line cabozantinib treatment compared with sunitinib in 157 patients with intermediate- or poor-risk metastatic RCC (8.2 vs 5.6 months; HR 0.66; 95% CI 0.46–0.95; P = 0.012) [54, 57].
More recently, Cabometyx® was demonstrated to improve OS and PFS in patients with advanced HCC who were previously treated with sorafenib (Nexavar®, Bayer, Whippany NJ, USA), according to data from the phase III CELESTIAL trial [58]. In this study, 707 patients with HCC were randomly assigned to receive cabozantinib or matching placebo, after failure of first-line sorafenib treatment. The results showed an improvement in median Overall Survival (mOS) (10.2 vs 8.0 months, HR 0.76; 95% CI 0.63–0.92; P = 0.005) and mPFS (5.2 vs 1.9 months, HR 0.44; 95% CI 0.36–0.52; P < 0.001) favoring cabozantinib. No novel safety signals were observed. Of note, cabozantinib was effective regardless of prior benefit from sorafenib. Based on these results, on May 29, 2018, the FDA accepted for filing a supplemental new drug application (sNDA) for Cabometyx® tablets for patients with previously treated advanced HCC.
Finally, cabozantinib is under investigation as single-agent or combined treatment in several other cancer types. A summary of the main trials of interest across different tumors and novel treatment combinations is reported in Table 2 [59,60,61,62].
4 Metabolic Profile of Approved Agents
4.1 Crizotinib
The pharmacokinetic and metabolic profiles of crizotinib after single and multiple administrations have been extensively characterized [63]. Following a single oral dose, crizotinib is absorbed with a median time to achieve peak concentration of 4–6 h and a half-life (t½) of approximately 42 h. At the dosage of 250 mg twice daily, the steady-state concentration was reached within 15 days of repeated administration, with a t½ of approximately 43–51 h [64]. The co-administration of crizotinib with a high-fat meal results in a non-clinically significant reduction in crizotinib exposure, with no change in other pharmacokinetic parameters [65]. Extra-renal excretion is the major route of drug elimination for this compound, with a high fecal excretion rate [66].
Crizotinib is predominantly metabolized in the liver by cytochrome P450 (CYP) 3A4/5 and aldehyde oxidase through two main pathways: oxidation of the piperidine ring to crizotinib lactam, and O-dealkylation. Of note, crizotinib exhibits non-linear pharmacokinetics which appear to be the result of crizotinib-mediated inhibition of CYP3A [64, 66]. The crizotinib lactam metabolite PF-06260182, the main circulating drug-related metabolite in plasma, still exhibits anti-ALK activity, although with a potency approximately three- to eightfold weaker than crizotinib [66].
Metabolic clearance of crizotinib is lower in patients with hepatic impairment than in patients with normal liver function, as demonstrated by a study of El-Khoueiry et al. [67]. However, clinical trials excluded patients with alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 2.5 × ULN (upper limit of normal), or > 5 × ULN in the case of liver metastases, as well as patients with total bilirubin of > 1.5 × ULN. Hence, additional data are needed to establish the optimal dose adjustment in patients with moderate hepatic impairment. Interestingly, a recent study reported a greater exposure to crizotinib and its active metabolite PF-06260182 in subjects with severe renal impairment (creatinine clearance < 30 mL/min) compared to those with normal renal function [68]. This is a consequence of a renal impairment-mediated decrease in hepatic metabolic clearance caused by a reduction in CYP3A enzymatical activity in these patients [69]. Conversely, mild/moderate renal impairment has no clinically relevant effect on exposure to crizotinib.
Crizotinib blood-to-plasma concentration ratio is approximately 1. In vitro studies suggested that crizotinib is a good transport substrate for P-glycoprotein (P-gp or ABCB1) and potential inhibitor for ATP-binding cassette transporters P-gp and breast cancer resistance protein (BCRP) [70, 71], which might influence the tissue distribution of the compound [72]. Furthermore, crizotinib has been identified as a substrate of the human hepatic uptake transport proteins organic anion-transporting polypeptides (OATPs) B1 and B3, but did not inhibit their activity in vitro at clinically relevant concentrations [73].
Age, body weight, ethnicity and gender did not affect exposure to crizotinib based on population pharmacokinetic analysis performed using pooled data from three trials of crizotinib in 1214 patients with ALK-positive advanced NSCLC and other solid tumors [74].
It is unknown whether crizotinib and its metabolites are excreted in human milk; hence lactation should be avoided during treatment.
4.2 Cabozantinib
The pharmacokinetics of cabozantinib have been extensively investigated in both healthy people and cancer patients [75]. As previously mentioned, cabozantinib is available in two different formulations: the capsule formulation (Cometriq®) is approved for treatment at a 140-mg free base equivalent dose, while the tablet formulation (Cabometyx®) is approved at a 60-mg free base equivalent dose [76]. Of note, bioequivalence could not be demonstrated between the cabozantinib capsule and tablet formulations following a single 140-mg dose in healthy subjects; in fact, a 19% increase in the maximum serum concentration (Cmax) of the tablet formulation compared to the capsule formulation was observed. Additionally, a less than 10% difference in the area under the concentration–time curve (AUC) was observed between cabozantinib tablet and capsule formulations [77].
Following oral administration, the peak cabozantinib plasma concentrations are reached at 3–4 h post-dose. Plasma concentration–time profiles show a second absorption peak approximately 24 h after administration, which suggests that cabozantinib may undergo enterohepatic recirculation. At the dosage of 140 mg twice daily, the steady-state concentration was reached within 15 days of repeated administration, with a t½ of approximately 99 h. Cabozantinib is highly protein bound in vitro in human plasma (≥ 99.7%). Based on the population pharmacokinetic model, the volume of distribution is approximately 319 L. It is mainly eliminated by the hepatobiliary system, as well as its six inactive metabolites, while urine excretion occurs only for metabolites. Cabozantinib exposure can be affected by CYP3A4 inducers and inhibitors, high-fat meals, hepatic impairment and minimally by renal failure [78]. In fact, cabozantinib AUC was increased by 63–81% or 7–30% in subjects with mild/moderate hepatic or renal impairment, respectively, and by 57% following a high-fat meal. Therefore, cabozantinib should not be taken with food, and should be used with caution in patients with mild/moderate hepatic impairment (recommended dosage in the case of moderate hepatic impairment = 40 mg once daily), avoiding its use in patients with severe renal and hepatic impairment. No specific dose adjustment is required in older people (≥ 65 years), while the safety and efficacy of cabozantinib in children and adolescents (< 18 years) have not been established. Of note, experience with cabozantinib in non-white patients is limited. Main drug interactions are discussed in Sect. 5.
5 Drug Interactions
Understanding drug–drug interactions (DDIs) is a critical step in the drug discovery process. Especially important are those DDIs mediated by CYP3A4, due to the great number of marketed drugs that are cleared by this enzyme [79]. As it happens with most TKIs, it is recommended to avoid co-administration of c-MET inhibitors with strong CYP3A4 inhibitors/inducers, such as carbamazepine, rifampin, rifabutin, rifapentine, and phenobarbital. As an example, administration of the strong CYP3A4 inhibitor ketoconazole to healthy subjects increased single-dose plasma cabozantinib exposure (AUC0–∞) by 38%. On the contrary, administration of the strong CYP3A4 inducer rifampin decreased single-dose plasma cabozantinib exposure by 77% [76]. Hence, in those cases where a strong CYP3A4 inhibitor/inducer is required, patients should be closely monitored, and a dose reduction of c-MET inhibitors may be required.
Crizotinib inhibits CYP3A both in vitro and in vivo, so its concomitant use together with CYP3A substrates with narrow therapeutic range, such as cyclosporine, ergotamine, fentanyl, quinidine, sirolimus, and tacrolimus, should be avoided, and if that is not possible, carefully monitored. Co-administration of crizotinib with strong CYP3A inhibitors like atazanavir, clarithromycin, itraconazole, ketoconazole, nelfinavir, ritonavir and voriconazole increases plasma concentrations of the c-MET inhibitor and is not recommended. Caution is recommended in the case of co-administration with moderate CYP3A4 inhibitors such as diltiazem or verapamil. The FDA also advises to avoid the intake of grapefruit or its juice, as it could increase the plasma concentrations of crizotinib. In addition, plasma concentrations of crizotinib can be decreased by co-administration with strong CYP3A inducers, such as carbamazepine, phenobarbital, phenytoin, rifabutin, rifampin, and St. John’s Wort. In vitro studies indicated that crizotinib is a weak inhibitor of uridine diphosphate glucuronosyltransferase (UGT) 1A1 and UGT2B7. However, clinical DDIs with UGT1A1 and UGT2B7 have been deemed unlikely to occur. Additionally, crizotinib inhibited P-gp, the hepatic uptake transporter organic cation transporter (OCT) 1, and renal uptake transporter, OCT2, in vitro at clinically relevant concentrations [11, 12, 63]. Hence, crizotinib has the potential to increase plasma concentrations of co-administered drugs that are substrates of these proteins, such as digoxin, colchicine, pravastatin, posaconazole, ranolazine, sitagliptin, tolvaptan, metformin and procainamide.
Cabozantinib is also a noncompetitive inhibitor of CYP2C8, a mixed-type inhibitor of CYP2C9 and CYP2C19, and a weak competitive inhibitor of CYP3A4, as observed in human liver microsomal (HLM) preparations. Furthermore, cabozantinib has been proven to act as an inducer of CYP1A1 messenger RNA in human hepatocytes. Additional clinical trials have been performed to determine whether the inhibition of CYP2C8 by cabozantinib observed in in vitro pre-clinical studies translates into potentially clinically significant DDIs. To evaluate this interaction, cabozantinib was co-administered with the antidiabetic drug rosiglitazone, a known CYP2C8 substrate, showing no effect on plasma exposure. In vitro data demonstrated that cabozantinib is a substrate of multidrug resistance-associated protein 2 (MRP2). Therefore, administration of MRP2 inhibitors such as cyclosporine, efavirenz and emtricitabine may increase cabozantinib plasma concentrations. In addition, cabozantinib is an inhibitor, but not a substrate, of P-gp transport activities [80]. Therefore, similarly to crizotinib, cabozantinib may potentially increase plasma concentrations of co-administered substrates of P-gp. Because of high plasma protein binding levels of cabozantinib, a displacement interaction with warfarin may be possible. Hence, international normalized ratio (INR) values should be monitored in the case of co-administration of these two drugs [14, 16].
The effect of cabozantinib on hormonal contraceptives has not been investigated, while the effectiveness of oral contraceptives may be reduced by concomitant crizotinib administration, and thus the use of additional contraceptive methods is recommended.
Dose adjustment of crizotinib or cabozantinib is not required when co-administrated with agents that increase gastric pH, such as proton inhibitors or antacids.
In clinical studies where c-MET inhibitors such as onartuzumab and cabozantinib were co-administered together with bevacizumab or erlotinib, no pharmacokinetic interactions were observed [81, 82].
A detailed list of DDIs for crizotinib and cabozantinib can be found online [83, 84].
6 Serious and Life-Threatening Labeled Adverse Reactions
6.1 Crizotinib
Toxicity data discussed below and in the next corresponding section (unless differently specified) reflect exposure to crizotinib in a total of 1722 patients comprising 1669 patients with ALK-positive advanced NSCLC enrolled in the aforementioned phase III PROFILE 1007 and PROFILE 1014 [28, 30] and single-arm PROFILE 1001 and PROFILE 1005 studies [26, 27], plus 53 patients with ROS1-positive advanced NSCLC [31]. These patients received crizotinib at a starting oral dosage of 250 mg taken twice daily continuously. In the case of grade 3 or 4 adverse events (AEs), as defined by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0, treatment dose reductions were generally applied according to the following schema: for grade 3 AEs, treatment was withheld until recovery to grade ≤ 2, then resumed at the same dose; for Grade 4 AEs treatment was withheld until recovery, then resumed at the next lower dose (first dose reduction to 200 mg twice daily, second dose reduction to 250 mg once daily, subsequent permanent discontinuation). Specific indications for dose reduction/discontinuation following severe selected toxicities are discussed in the following sections [11, 12].
The most frequently observed serious and life-threatening AEs across different trials were hepatotoxicity, interstitial lung disease (ILD)/pneumonitis, neutropenia, and QT interval prolongation.
6.1.1 Hepatotoxicity
Crizotinib-induced hepatotoxicity with fatal outcome was observed in 0.1% of patients. Grade 3 or 4 transaminase elevations were observed in 11% and 6% of patients, respectively. Concurrent elevation in ALT and/or AST ≥ 3 × ULN and total bilirubin ≥ 2 × ULN without significant elevations of alkaline phosphatase (ALP) have been observed in < 1% of patients. Transaminase elevations generally occurred within the first 2 months of treatment and were manageable by dose interruption and modifications. Of note, crizotinib treatment should be discontinued in the case of concomitant grade 2 or higher AST and/or ALT and total bilirubin elevation in the absence of cholestasis or hemolysis.
The recommended starting dose of crizotinib for patients with baseline mild hepatic impairment is 200 mg twice daily. The starting dose for patients with baseline moderate/severe hepatic impairment is recommended to be 250 mg once daily. Monitoring of liver function tests including ALT, AST, and total bilirubin is recommended weekly during the first 2 months of treatment, followed by once a month or as clinically indicated, with more frequently repeated testing in patients who develop liver function alterations.
6.1.2 Interstitial Lung Disease/Pneumonitis
Severe, life-threatening, or fatal ILD/pneumonitis occurred in 0.5% of patients treated with crizotinib. ILD of any grade was observed in 3% of patients and grade 3 or 4 in 1.0% of cases. These AEs generally occurred within the first 3 months of treatment. Patients with pulmonary symptoms indicative of ILD/pneumonitis should be monitored, and crizotinib administration should be withheld if this AE is suspected. Crizotinib should be permanently discontinued following a diagnosis of treatment-related ILD/pneumonitis (any grade).
6.1.3 QT interval prolongation
QTc prolongation ≥ 500 ms has been reported in 2.1% of patients with at least one post-baseline electrocardiogram (ECG) assessment across different trials (N = 1619), and a maximum increase from baseline in QTc of ≥ 60 ms was observed in 5.0% of 1585 patients with a baseline and at least one post-baseline ECG assessment. This AE is associated with an increased risk of ventricular tachyarrhythmias or sudden death. All-causality grade 3 or 4 QT prolongation was reported in 1.6% of patients. In a dedicated single-arm ECG study, the largest mean change from baseline in QTc was 12.3 ms (95% CI 5.1–19.5) observed at 6 h post-dose on cycle 2 day 1.
A careful assessment of the benefits and potential risks of crizotinib should be performed in patients with pre-existing conditions such as bradycardia, a history of or predisposition for QTc prolongation, relevant pre-existing cardiac disease and/or electrolyte disturbances or in those who are taking antiarrhythmics or other drugs that are known to prolong QT interval. Crizotinib should be administered with caution in these patients, and periodic monitoring of ECG, electrolytes and renal function is required. Close management of any potential AE which may contribute to increase the risk of this type of toxicity, i.e., vomiting, diarrhea, dehydration or impaired renal function, is recommended.
Crizotinib should be withheld and cardiologist advice should be prompted in the case of a QTc increase ≥ 60 ms from baseline when QTc is < 500 ms. If QTc increases to ≥ 500 ms, an immediate cardiological evaluation is mandatory. Crizotinib should be permanently discontinued in patients who developed a QTc of ≥ 500 ms or a change from baseline of ≥ 60 ms with torsade de pointes or polymorphic ventricular tachycardia or signs/symptoms of serious arrhythmia.
6.1.4 Bradycardia
Symptomatic bradycardia (i.e., syncope, dizziness, hypotension) can occur in patients treated with crizotinib and have been observed in 2.4% of patients across clinical trials, while all-causality bradycardia was experience by up to 13% of patients. This AE appears to be dose-dependent, with an average decrease of heart rate of 2.5 beats/min every 100 ng/mL increase in crizotinib concentration [85]. The full effect of crizotinib on the reduction of heart rate may develop several weeks after the start of treatment; thus heart rate and blood pressure should be monitored regularly. Dose modification is not required in cases of asymptomatic bradycardia, while crizotinib should be withheld until recovery in the case of non-life-threatening symptomatic bradycardia and permanently discontinued in the case of life-threatening drug-mediated bradycardia in the absence of concomitant medications known to cause bradycardia or hypotension. If concomitant medications can be adjusted or discontinued, crizotinib can be restarted at 250 mg once daily upon symptom resolution, with frequent monitoring.
The use of crizotinib in combination with other bradycardic agents such as beta-blockers, non-dihydropyridine calcium channel blockers (i.e., verapamil and diltiazem), clonidine, anticholinesterases, pilocarpine and digoxin should be avoided to the maximum extent possible.
6.1.5 Cardiac Failure
Cases of severe, life-threatening or fatal cardiac failure were reported in clinical studies with crizotinib and during post-marketing surveillance. Patients should be monitored for signs and symptoms of heart failure during crizotinib treatment regardless of previous history of cardiac disorders. Dose reductions, treatment suspension or discontinuation should be considered as appropriate if such symptoms are observed.
6.1.6 Neutropenia and Leukopenia
Across studies in patients with either ALK-positive or ROS1-positive advanced NSCLC, grade 3 or 4 neutropenia has been commonly reported (12%). However, less than 0.5% of patients experienced febrile neutropenia. Neutropenia was associated with dose reduction or permanent treatment discontinuation in 3% and < 1% of patients, respectively. Median time to onset of any grade neutropenia was 89 days. Grade 3 or 4 leukopenia was observed in 3% of patients, with a similar median time to onset (85 days, any grade). Leukopenia was associated with a dose reduction for < 0.5% of patients, and no patients permanently discontinued treatment.
Complete blood counts including differential white blood cell counts should be monitored monthly and as clinically indicated, with more frequent testing in the case of grade 3 or 4 alterations, fever or infection.
6.1.7 Gastrointestinal Perforation
GI perforation was reported as a severe AE in clinical studies with crizotinib, and cases of fatal GI perforation were reported during the post-marketing use of this agent. Crizotinib should be used with caution in patients at higher risk for GI perforation, and treatment should be discontinued in patients who develop this AE. Patients should be advised to promptly seek medical attention in the case of occurrence of GI perforation symptoms.
6.1.8 Severe Visual Loss
Grade 4 visual field defects with severe vision loss have been reported in 0.2% of patients treated with crizotinib, possibly due to optic atrophy and optic nerve disorders. All-grade vision disorders, most commonly visual impairment, vision blurred, photopsia and vitreous floaters, were experienced by 63% of patients, although in 95% of cases these AEs were not serious. 0.4% of patients temporarily discontinued treatment, and 0.1% had a dose reduction associated with vision disorders. The onset of these disorders generally started within the first week of treatment. Crizotinib should be discontinued in patients with new onset of grade 4 visual loss, defined as best corrected visual acuity of < 1/10 in one or both eyes. Ophthalmological evaluation should be performed upon the occurrence of this AE. The eventual decision to resume crizotinib treatment should be based on a careful evaluation of the potential benefit for the patient, as insufficient evidence is available to define the risks of resumption of crizotinib in patients with a severe visual loss. Ophthalmological evaluation is recommended if vision disorders persist or worsen.
6.1.9 Renal Failure
Renal failure and acute renal failure were reported in patients treated with crizotinib across different clinical trials (< 1%) and during post-marketing surveillance. Cases requiring hemodialysis and cases with fatal outcome were also observed. Hence, monitoring of patients for renal function at baseline and during treatment is recommended.
The crizotinib starting dosage should be adjusted to 250 mg once daily in patients with severe renal impairment (creatinine clearance < 30 mL/min) not requiring peritoneal dialysis or hemodialysis. The dosage may be subsequently increased to 200 mg twice daily after at least 4 weeks of treatment based on individual safety and tolerability.
6.1.10 Summary
In summary, the most frequent AEs (≥ 3%, all-causality frequency) associated with treatment interruptions and dose reductions were neutropenia and elevated transaminases. Permanent treatment discontinuation due to AEs occurred in 18% of patients, most frequently due to ILD (1%) and elevated transaminases (1%). Fatal AEs in addition to those already discussed included respiratory distress syndrome, arrhythmia, pulmonary embolism, respiratory failure, and sepsis.
6.2 Cabozantinib
As aforementioned, Cabometyx® tablets and Cometriq® capsules are not bioequivalent and should not be used interchangeably. Therefore, it is recommended that if a patient must switch from cabozantinib capsules to cabozantinib tablets, the patient should continue at a Cabometyx® dose that does not exceed 60 mg or the current Cometriq® dose.
The recommended dosage of Cabometyx® is 60 mg once daily, while the recommended dosage for Cometriq® is 140 mg once daily (taken as one 80-mg orange capsule and three 20-mg grey capsules). In the case of AEs, as defined by NCI CTCAE version 4.0, it is recommended to reduce Cabometyx® to 40 mg daily and then to 20 mg daily, while Cometriq® needs to be reduced to 100 mg daily (taken as one 80-mg orange capsule and one 20-mg grey capsule) and then to 60 mg daily (taken as three 20-mg grey capsules). Treatment dose reductions are generally applied according to the following schema: for grade 3 and 4 AEs, treatment is withheld until recovery to grade 1, then resumed at a reduced dose. It is recommended to permanently discontinue cabozantinib treatment in the case of development of unmanageable fistula or GI perforation, severe hemorrhage, arterial thromboembolic event, hypertensive crisis or severe hypertension despite optimal medical management, nephrotic syndrome, reversible posterior leukoencephalopathy syndrome (RPLS), or osteonecrosis of the jaw (ONJ) (for Cometriq®).
Safety data on Cabometyx® derive from the two randomized clinical trials in advanced RCC patients: in the METEOR study, dose reductions and dose interruptions due to an AE occurred in 59.8% and 70%, respectively; in treatment-naïve RCC (CABOSUN trial), dose reductions and dose interruptions occurred in 46% and 73%, respectively.
Safety data on Cometriq® are based on the pivotal trial on MTC patients [50].
The most frequently observed serious and life-threatening AEs were perforations and fistulas, hemorrhage, thromboembolic events, wound complications, palmar-plantar erythrodysaesthesia syndrome (PPES), ONJ, and RPLS.
6.2.1 Perforations, Fistulas, and Intra-abdominal Abscesses
Serious GI perforations and fistulas, sometimes fatal, and intra-abdominal abscesses have been observed with cabozantinib. Fistulas were reported in 1.2% and GI perforations were reported in 0.9% of Cabometyx®-treated patients, while in Cometriq®-treated patients, GI perforations and fistulas were reported in 3% and 1%, respectively.
6.2.2 Thromboembolic Events
The use of cabozantinib has been associated with venous thromboembolism, including pulmonary embolism, and arterial thromboembolism.
With Cabometyx® treatment, venous thromboembolism was reported in 7.3% and pulmonary embolism in 3.9% of patients, while with Cometriq®, venous thromboembolism occurred in 6% and arterial thromboembolism in 2% of patients.
Cabozantinib must be discontinued in patients who develop acute myocardial infarction or any other arterial thromboembolic complication.
6.2.3 Hemorrhage, Hypertension and Wound Complications
In patients treated with Cabometyx®, hypertension was reported in 37% of cases (15% grade ≥ 3). The incidence of grade ≥ 3 hemorrhagic events was 2.1%.
In patients treated with Cometriq®, grade 1 or 2 hypertension was reported in 61% of cases. The incidence of grade ≥ 3 hemorrhagic events was higher in Cometriq®-treated patients compared with patients receiving placebo (3% vs 1%). Of note, serious and sometimes fatal hemorrhages also occurred in the cabozantinib clinical program.
Cabozantinib treatment should be stopped at least 28 days prior to scheduled surgery and should be discontinued in patients with wound healing complications requiring medical intervention.
6.2.4 Palmar-Plantar Erythrodysaesthesia Syndrome
PPES occurred in 50% of patients treated with Cometriq® (13% grade ≥ 3) and in 42% of patients treated with Cabometyx® (8.2% grade ≥ 3). In the case of grade ≥ 3 PPES, cabozantinib should be withheld until improvement to grade 1, and then resume at a reduced dose.
6.2.5 Osteonecrosis of the Jaw
ONJ can manifest as jaw pain, osteomyelitis, osteitis, bone erosion, tooth or periodontal infection, toothache, gingival ulceration or erosion, persistent jaw pain or slow healing of the mouth or jaw after dental surgery. ONJ occurred in 1% of Cometriq®-treated patients.
6.2.6 Reversible Posterior Leukoencephalopathy Syndrome
RPLS usually evolves in a few hours with the most common presenting symptoms being seizures, disturbed vision, headache, and altered mental state. It occurred in < 1% of patients treated with cabozantinib.
6.2.7 QT interval prolongation
The effect of cabozantinib on QTc interval was evaluated in a randomized, double-blinded, placebo-controlled study in patients with MTC at a dosage of 140 mg once daily. A mean increase in QTc of 10–15 ms was observed at 4 weeks after treatment start. However, this effect was not associated with a change in cardiac wave morphology or new rhythms. In addition, a concentration–QTc relationship could not be definitively established. Therefore, cabozantinib should be used with caution in patients with a history of QT interval prolongation, patients who are taking antiarrhythmics, or patients with relevant pre-existing cardiac disease, bradycardia, or electrolyte disturbances.
7 Most Frequent Other Labeled Adverse Reactions
7.1 Crizotinib
The most common AEs (≥ 25%) in patients with either ALK-positive or ROS1-positive advanced NSCLC were vision disorders, nausea, diarrhea, vomiting, edema, constipation, elevated transaminases, fatigue, decreased appetite, upper respiratory infection, dizziness, and neuropathy [11, 12].
Of note, in recent ALCL trials, no novel safety concerns were reported. Across the adult population, the most common all-grade AEs related to treatment were diarrhea (45.5%) and vision disorders (45.5%) [33, 34]. In the pediatric population, the most frequently reported all-grade AE was a decrease in neutrophil count, which occurred in 33% of patients receiving the lower dose of crizotinib and 70% of those receiving the higher dose.
7.1.1 Gastrointestinal Effects
The most commonly reported all-causality GI AEs were nausea (57%), diarrhea (54%), vomiting (51%), and constipation (43%). Most of these AEs were mild to moderate. Median time to onset for nausea and vomiting was 3 days, and their incidence declined after 3 weeks of treatment. Standard symptomatic and supportive care was effective in the management of these AEs. Among the most frequent AEs associated with treatment interruptions, vomiting and nausea were reported in 5% and 4% of cases, respectively.
7.1.2 Peripheral Edema
Treatment with crizotinib has been associated with the development of peripheral edema in 47% of patients. This appears to be a cumulative, late-onset effect and is usually well-managed with diuretics and compression stockings when appropriate.
7.1.3 Endocrine Disorders
Suppression of testosterone levels (secondary hypogonadism) in men during crizotinib treatment has been reported in 2% of patients. Hormonal levels have been observed to normalize after treatment discontinuation. The role of hormone replacement upon the occurrence of this AE in asymptomatic patients is unclear.
7.1.4 Nervous System Effects
All-causality neuropathy was reported in 25% of patients treated with crizotinib. Dysgeusia was also reported in 21% of patients, primarily grade 1.
7.1.5 Dermatologic Toxicity
In clinical trials, grade 1 or 2 rash was reported to occur in 13% of patients. However, a 2014 case report described a crizotinib-related grade 4 photosensitive rash resolving after treatment discontinuation [86], and more recently a fatal case of toxic epidermal necrolysis during crizotinib treatment was reported [87]; thus monitoring for these AEs is advised.
7.1.6 Renal Cyst
All-causality complex renal cysts development was reported in 3% of patients across different trials. Of note, local cystic invasion beyond the kidney was observed in some cases. Periodic monitoring with imaging and urine analysis is advised upon the occurrence of this AE.
7.2 Cabozantinib
The most common AEs (≥ 25%) in patients with advanced RCC treated with Cabometyx® included, in order of decreasing frequency, diarrhea, fatigue, nausea, decreased appetite, PPES, hypertension, vomiting, weight loss, and constipation. Grade 3 or 4 AEs and laboratory abnormalities which occurred in ≥ 5% of patients were hypertension, diarrhea, fatigue, PPES, hyponatremia, hypophosphatemia, hypomagnesemia, lymphocyte decrease, anemia, hypokalemia, and gamma-glutamyl transferase (GGT) increase.
A slightly different safety profile has been observed for patients with MTC treated with Cometriq®, whose most commonly reported AEs (≥ 25%) have been, in order of decreasing frequency, diarrhea, stomatitis, PPES, weight loss, decreased appetite, nausea, fatigue, oral pain, hair color changes, dysgeusia, hypertension, abdominal pain, and constipation.
The most frequent laboratory abnormalities (> 25%) were increased AST/ALT, lymphopenia, increased ALP, hypocalcemia, neutropenia, thrombocytopenia, hypophosphatemia, and hyperbilirubinemia. Grade 3 or 4 AEs and laboratory abnormalities occurring in ≥ 5% of patients included diarrhea, PPES, lymphopenia, hypocalcemia, fatigue, hypertension, asthenia, increased ALT, weight loss, stomatitis, and decreased appetite.
8 Post-marketing Spontaneous Reports Data on FAERS, EudraVigilance and Regulatory Alerts
After first FDA approval in 2011, a total of 4617 serious AEs have been reported for crizotinib as of June 2018, including 2047 death cases, according to the FDA Adverse Reporting System (FAERS) Public Dashbord (available online) [88]. As for cabozantinib, the FEARS database reports 2749 serious events since 2013, including 1085 death cases.
Reported events were consistent with previously discussed evidence from clinical trials and known drug-related toxicities, and pertinent warnings and precautions are reflected in the corresponding drug label information of both agents.
Procedural steps taken and scientific information/regulatory alerts after marketing authorization by the EMA for crizotinib and cabozantinib can be found online as part of periodically updated EPAS (European public assessment reports) [89,90,91].
9 New Agents Under Development
9.1 Monoclonal Antibodies
Rilotumumab (AMG 102, Amgen, Thousand Oaks, California, USA) is a fully human HGF-neutralizing mAb. The efficacy of this agent in combination with chemotherapy has been evaluated in two placebo-controlled, randomized phase III clinical trials, RILOMET-1 and 2, for patients with previously untreated HER2-negative c-MET-positive advanced gastric or gastroesophageal junction (GEJ) cancer. A significantly shorter OS in the experimental arms compared to the standard-of-care arms, due to an imbalance in deaths resulting from disease progression, prompted an early closure of both clinical studies [92, 93]. A phase Ib/II study evaluating rilotumumab versus panitumumab in patients with previously treated KRAS wild-type metastatic CRC suggested a benefit from combining rilotumumab and panitumumab in this setting [94]. Rilotumumab has also been evaluated in a phase II trial aiming to study its safety and efficacy in combination with bevacizumab (Avastin®, Genentech, South San Francisco, California, USA) for the treatment of recurrent malignant glioma, showing no significant improvement and low tolerability [95]. A phase I/II trial investigated the efficacy of rilotumumab in combination with erlotinib in previously treated metastatic NSCLC. No dose-limiting toxicities were observed, and six out of eight enrolled patients showed disease control (three PR plus three SD) [96]. However, no further clinical investigations are undergoing in this setting at the moment.
Ficlatuzumab (AV-229, AVEO Pharmaceuticals, Cambridge, Massachusetts, USA), another HGF-neutralizing mAb, is currently being studied in squamous cell carcinoma of the head and neck (HNSCC), metastatic pancreatic ductal cancer (PDAC), and acute myeloid leukemia (AML). Of note, the combination of this agent with gefitinib (Iressa®, AstraZeneca, Cambridge, UK) in the treatment of Asian patients with NSCLC failed to provide significant improvement over gefitinib monotherapy in two phase II trials [97, 98]. A phase II study investigating the combination of erlotinib with ficlatuzumab in untreated metastatic EGFR-mutant NSCLC is ongoing (NCT02318368).
Among the mAbs that bind to the extracellular domain of c-MET, the fully humanized monovalent mAb onartuzumab (MetMAb®, Genentech, South San Francisco, California, USA) has been evaluated in several clinical trials, but failed to show improvement when given in combination with erlotinib in patients with MET-positive NSCLC [21, 22, 99]. Similarly, both the phase III METGastric trial, evaluating the addition of onartuzumab to first-line chemotherapy with mFOLFOX6 (oxaliplatin, 5-fluorouracil and leucovorin) in HER2-negative, c-MET-positive metastatic gastric or GEJ cancers, and a randomized phase II study, investigating the activity of onartuzumab plus first-line FOLFOX-bevacizumab in CRC, failed to demonstrate significant results [100, 101].
Emibetuzumab (LY2875358, Eli Lilly and Company, Indianapolis, Indiana, USA) is a bivalent humanized anti–c-MET mAb that blocks HGF/c-MET interaction and promotes c-MET internalization and degradation. A recent first-in-human phase I clinical study showed encouraging safety and efficacy results of this agent as both single-agent treatment in patients with solid tumors and in combination with erlotinib in patients with NSCLC [102]. Phase II clinical studies of emibetuzumab in EGFR-mutant NSCLC and in combination with the anti-VEGFR2 mAb ramucirumab (Cyramza®, Eli Lilly and Company) are ongoing (NCT01897480 and NCT02082210, respectively).
ARGX-111 is an anti–c-MET IgG1 antibody characterized by improved tissue penetration and enhanced antibody-dependent cellular cytotoxicity [103]. This agent has been recently evaluated in a phase I clinical trial in patients with advanced cancers overexpressing c-MET (NCT02055066), showing a good safety profile [104]. Publication of final safety and efficacy results of this trial is pending.
Other anti-MET mAbs in development are YYB-101 (YooYoung Pharmaceutical Co, Seoul, Korea), a humanized rabbit anti-HGF antibody currently being tested in an ongoing phase I trial in patients with advanced solid tumors (NCT02499224); JNJ-61186372, a bispecific anti-EGFR/c-MET mAb, under study in a phase I clinical trial in advanced NSCLC (NCT02609776); and ABT-700, a bivalent humanized IgG1, which revealed single-agent activity in a phase I trial in MET-amplified gastric or GEJ cancers [105], and is currently being tested in a phase I study in combination with chemotherapy in patients with MET-amplified/c-MET overexpressing advanced solid tumors (NCT01472016). Additionally, telisotuzumab vedotin (Teliso-V, ABBvie, North Chicago, Illinois, USA), formerly ABBV-399, a novel, first-in-class antibody–drug conjugate of ABT-700 and monomethyl auristatin E, showed promising safety and efficacy results in patients with c-MET-positive NSCLC, both as single-agent and in combination with erlotinib [106, 107], and is currently under study in combination with nivolumab as second line-treatment of advanced c-MET overexpressing NSCLC (NCT03539536).
9.2 Small Molecule c-MET Kinase Inhibitors
Capmatinib (INC280, Novartis, Basel, Switzerland) is a potent oral, ATP-competitive, class I c-MET inhibitor. The efficacy and safety of this molecule as a single agent were investigated in a phase 1 trial in patients with advanced solid tumors, including 55 EGFR wild-type c-MET-positive NSCLC patients [108]. Based on phase I encouraging results, a phase II trial of capmatinib in NSCLC harboring c-MET exon 14 skipping mutation is currently ongoing (NCT03693339). Additionally, another phase II study is investigating NSCLC patients with c-MET exon 14 alterations who have received a prior c-MET inhibitor (NCT02750215). Capmatinib has also been evaluated in combination with gefitinib in patients with EGFR-mutant NSCLC after progression to gefitinib treatment [109]. The ORR was 50% in patients with MET amplification (gene copy number ≥ 6). Similar results were observed in a small study with the combination of capmatinib and erlotinib. A phase II trial is currently investigating the safety and efficacy of capmatinib as a single agent or in combination with erlotinib compared to chemotherapy in advanced/metastatic EGFR-mutated c-MET-amplified NSCLC (NCT02468661). Other settings of clinical development of capmatinib include the treatment of advanced HCC (NCT02795429), papillary RCC (NCT02019693) and melanoma, as well as the combination with novel EGFR-inhibitors and immunotherapy in NSCLC (NCT02335944, NCT02323126).
Tepotinib (EMD 1214063, Merck, Darmstadt, Germany) is a highly selective first class c-MET TKI, which has recently showed anti-tumor clinical activity in an ongoing phase II study with NSCLC patients harboring c-MET exon 14 skipping mutations, as informed during the 2018 American Society of Clinical Oncology (ASCO) annual meeting [110]. Additionally, promising preliminary results have been shown in early phase trials in c-MET-positive EGFR-mutant NSCLC in combination with gefitinib (NCT01982955) and in HCC [111].
Savolitinib (AZD6094/HMPL-504, AstraZeneca, Cambridge, UK), another class I c-MET inhibitor, is currently being studied in multiple tumor types, including central nervous system tumors, RCC, NSCLC, and GI cancers, both as monotherapy and in combination with other targeted and immunotherapy agents.
Another recent example of a class I c-MET inhibitor in earlier phases of clinical development is AMG-337 (Amgen, Thousand Oaks, California, USA), an orally bioavailable highly selective inhibitor of the c-MET receptor with a half maximal inhibitory concentration (IC50) of 1 nM which has reached phase II studies in patients with GI cancer or other MET-amplified solid tumors [112].
Numerous class II inhibitors have been developed as well. Foretinib (GSK1363089, GlaxoSmithKline, Brentford, UK) is an oral multi-targeted kinase inhibitor of c-MET, c-ROS, RON/macrophage stimulating 1 receptor (MST1R), AXL, TIE-2, and VEGFR2/3. This agent has been evaluated in several phase I and II clinical trials in patients with breast cancer, NSCLC, HNSCC, RCC, gastric cancer, and other solid tumors [113,114,115,116,117].
Merestinib (LY2801653, Eli Lilly and Company, Indianapolis, Indiana, USA) is an orally bioavailable c-MET inhibitor that targets several other kinases like RON, FLT3, AXL, MER proto-oncogene tyrosine kinase (MERTK), TIE-2, ROS1, neurotrophic tropomyosin receptor kinase (NTRK) 1/2/3, discoidin domain receptor 1 and 2 (DDR1/2), and MAP kinase interacting serine/threonine kinase 1 and 2 (MKNK1/2) [118, 119], and is currently under early phases of clinical development (NCT02920996).
Sitravatinib (MGCD516, Mirati Therapeutics, San Diego, California, USA) binds and blocks the activity of several kinases beyond c-MET, including AXL, MERTK, VEGFR1/2/3, DDR2, and members of the tropomyosin-related kinases (TRK), platelet-derived growth factor receptor (PDGFR), and ephrin (Eph) families, among others. It is now under clinical evaluation to determine its safety and efficacy in patients with RCC, soft tissue sarcomas, NSCLC, HNSCC, urothelial carcinoma and other solid tumors.
Also from Mirati Therapeutics, glesatinib (MGCD265) potently and selectively targets tumors with driver alterations in MET and AXL, and is currently being evaluated in a phase II trial in NSCLC patients exhibiting an activating genetic alteration of MET (NCT02544633). Additionally, a phase II study of glesatinib in combination with nivolumab in NSCLC is ongoing (NCT02954991).
BMS-777607 (ASLAN-002, ASLAN Pharmaceuticals/Bristol-Myers Squibb Co., Singapore), an inhibitor of c-MET, AXL, RON and tyrosine-protein kinase receptor TYRO3, and BMS-794833, a potent inhibitor of c-MET, VEGFR2, RON, AXL, and FLT3 that serves as a prodrug for BMS-817378, showed IC50 values in the low nanomolar range and reached clinical trial evaluation for advanced solid tumors.
TAS-115 (Taiho Pharmaceutical CO., LTD., Tokyo, Japan) is a c-MET and VEGFR2 inhibitor, which has also demonstrated inhibitory activity of the feline McDonough sarcoma oncogene (FMS) kinase, essential for the differentiation of osteoclasts [120, 121]. It is currently under phase I/II clinical trials in Japan to study its safety and efficacy in castration-resistant prostate cancer patients with bone metastases [122].
S49076 (Servier, Neuilly-sur-Seine, France) is an oral ATP-competitive inhibitor of c-MET, AXL, and fibroblast growth factor receptors 1/2/3 (FGFR1/2/3) which has been evaluated in phase I/II studies for HCC and NSCLC [123, 124]. Another potent multi-target inhibitor of c-MET, RON, VEGFR1, FGFR1/2/3, PDGFR, and other kinases is MK-2461 (Merck), which reached phase I and II studies in patients with advanced solid tumors.
Finally, the orally bioavailable class III c-MET allosteric inhibitor tivantinib (ARQ197, Daiichi-Sankyo, Tokyo, Japan), which binds to an inactive dephosphorylated c-MET conformation, stabilizing it and hence blocking its downstream signaling [20, 125], has been extensively investigated in several cancer types, including HCC, CRC, NSCLC, breast, and GI cancers [126], although results have been disappointing so far. In a recent phase II study [127], tivantinib showed promising results in patients with advanced HCC and Child–Pugh class A cirrhosis, improving mPFS (2.7 months vs 1.4 months, P = 0.03) compared with placebo in a subset of patients with high MET expression (MET-high). However, the phase III METIV-HCC trial did not confirm these results in patients with MET-high HCC who had progressed or were intolerant to sorafenib, randomly assigned to receive tivantinib (120 mg twice daily) or placebo [128]. No benefit was observed from tivantinib treatment, with a median OS of 8.4 months in the tivantinib group compared to 9.1 months in the placebo group (HR 0.97; 95% CI 0.75–1.25; P = 0.81) [128]. The MARQUEE phase III trial tested the efficacy of tivantinib (360 mg twice daily) plus erlotinib compared to erlotinib plus placebo in 1048 patients with advanced NSCLC previously treated with one or two systemic regimens [129]. The study was discontinued for futility at the interim analysis since no significant OS benefit was observed, although mPFS was significantly improved in the tivantinib group (3.6 vs 1.9 months in the placebo group; HR 0.74; P < 0.001). Notably, exploratory analyses suggested that OS improved in patients with high MET expression (9.3 vs 5.9 months; HR 0.70; 95% CI 0.49–1.01), which was also noted in the HCC trials. In an exploratory subgroup analysis of the MARQUEE trial including 109 patients with EGFR-mutant disease, the combination of tivantinib plus erlotinib improved mPFS (13.0 vs 7.5 months in the placebo group; HR 0.49; 95% CI 0.31–0.77) and OS (25.5 vs 20.3 months; HR 0.68; 95% CI 0.43–1.08) [130]. Tivantinib is currently under development in other settings, and results from phase II–III trials are eagerly awaited [131].
Most common AEs reported in clinical trials with main novel c-MET inhibitors previously discussed are reported in Table 3.
10 Conclusions
The HGF/MET pathway plays a pivotal role in cancer and represents an attractive treatment target in several malignancies. Over the past decade, great effort has been dedicated to the development of novel selective targeted c-MET inhibitors, either small molecules or mAbs, which have demonstrated meaningful preliminary clinical efficacy and an overall manageable toxicity profile. However, to date, results of most phase III trials have been negative. Currently approved c-MET inhibitors, crizotinib and cabozantinib, have greatly contributed to the therapeutic options in selected tumor types. Most frequent AEs are generally mild/moderate, with distinct profiles for each agent. Post-marketing safety monitoring of the increasing patient population receiving c-MET inhibitors and ongoing studies testing combination strategies with other targeted agents or chemotherapeutic drugs are of paramount importance to further assess MET inhibitors tolerability and safety and implement toxicity-preventing strategies.
The dissection of the multiple mechanisms at the basis of c-MET pathway activation and primary and acquired resistance to c-MET inhibition in different tumor types will lead to further improvement in targeted drug development and patient selection to optimize outcome and toxicity exposure. Of note, FDA approval of crizotinib requires a companion diagnostic limiting its administration to molecularly selected tumors, including for the first time in 2018, c-MET exon 14 alterations in NSCLC. In fact, although most trials across different cancer types have been restricted to c-MET overexpressing tumors identified by different techniques (i.e., immunohistochemistry or FISH), the diagnostic tests and molecular predictive biomarkers to identify patients who may benefit from c-MET inhibition are still not well understood. High throughput next generation sequencing (NGS) technologies and novel testing strategies are currently being exploited to implement patient selection and support clinical trial design and targeted anti-MET drug development.
References
Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34.
Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373(6516):699–702.
Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, et al. c-Met is essential for wound healing in the skin. J Cell Biol. 2007;177(1):151–62.
Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373(6516):702–5.
Boccaccio C, Comoglio PM. MET, a driver of invasive growth and cancer clonal evolution under therapeutic pressure. Curr Opin Cell Biol. 2014;31:98–105.
Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):45.
Cappuzzo F, Marchetti A, Skokan M, Rossi E, Gajapathy S, Felicioni L, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27(10):1667–74.
Zhao X, Qu J, Hui Y, Zhang H, Sun Y, Liu X, et al. Clinicopathological and prognostic significance of c-Met overexpression in breast cancer. Oncotarget. 2017;8(34):56758–67.
Garajova I, Giovannetti E, Biasco G, Peters GJ. c-Met as a target for personalized therapy. Transl Oncogenom. 2015;7(Suppl 1):13–31.
Ko B, He T, Gadgeel S, Halmos B. MET/HGF pathway activation as a paradigm of resistance to targeted therapies. Ann Transl Med. 2017;5(1):4.
Crizotinib (Xalkori®) FDA label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202570s021lbl.pdf. Accessed 4 Octob 2018.
Crizotinib (Xalkori®) EMA label. https://www.ema.europa.eu/documents/product-information/xalkori-epar-product-information_en.pdf. Accessed 4 Oct 2018.
Cabozantinib (Cometriq®) FDA label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203756lbl.pdf. Accessed 4 Oct 2018.
Cabozantinib (Cometriq®) EMA label. https://www.ema.europa.eu/documents/product-information/cometriq-epar-product-information_en.pdf. Accessed 4 Oct 2018.
Cabozantinib (Cabometyx®) FDA label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208692s000lbl.pdf. Accessed 4 Oct 2018.
Cabozantinib (Cabometyx®) EMA label: https://www.ema.europa.eu/documents/product-information/cabometyx-epar-product-information_en.pdf. Accessed 4 Oct 2018.
Qiu P, Wang S, Liu M, Ma H, Zeng X, Zhang M, et al. Norcantharidin inhibits cell growth by suppressing the expression and phosphorylation of both EGFR and c-Met in human colon cancer cells. BMC Cancer. 2017;17(1):55.
Hughes VS, Siemann DW. Have clinical trials properly assessed c-Met inhibitors? Trends Cancer. 2018;4(2):94–7.
Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(1 Suppl):S7–19.
Parikh PK, Ghate MD. Recent advances in the discovery of small molecule c-Met Kinase inhibitors. Eur J Med Chem. 2018;143:1103–38.
Mo H-N, Liu P. Targeting MET in cancer therapy. Chronic Dis Transl Med. 2017;3(3):148–53.
Goździk-Spychalska J, Szyszka-Barth K, Spychalski Ł, Ramlau K, Wójtowicz J, Batura-Gabryel H, et al. c-MET inhibitors in the treatment of lung cancer. Curr Treat Options Oncol. 2014;15(4):670–82.
Bouattour M, Raymond E, Qin S, Cheng AL, Stammberger U, Locatelli G, et al. Recent developments of c-Met as a therapeutic target in hepatocellular carcinoma. Hepatology (Baltimore, MD). 2018;67(3):1132–49.
Ou S-HI, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thoracic Oncol. 2011;6(5):942–6.
Crinò L, Kim D, Riely GJ, Janne PA, Blackhall FH, Camidge DR, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29(15_suppl):7514.
Blackhall F, Ross Camidge D, Shaw AT, Soria JC, Solomon BJ, Mok T, et al. Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open. 2017;2(3):e000219.
Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Solomon BJ, Mok T, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77.
Solomon BJ, Kim D-W, Wu Y-L, Nakagawa K, Mekhail T, Felip E, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non–small-cell lung cancer. J Clin Oncol. 2018;36(22):2251–8.
Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–71.
Drilon AE, Camidge DR, Ou S-HI, Clark JW, Socinski MA, Weiss J, et al. Efficacy and safety of crizotinib in patients (pts) with advanced MET exon 14-altered non-small cell lung cancer (NSCLC). J Clin Oncol. 2016;34(15_suppl):108.
Gambacorti-Passerini C, Orlov S, Zhang L, Braiteh F, Huang H, Esaki T, et al. Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): a phase 1b open-label study. Am J Hematol. 2018;93(5):607–14.
Mossé YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children’s Oncology Group Study. J Clin Oncol. 2017;35(28):3215–21.
Schoffski P, Wozniak A, Kasper B, Aamdal S, Leahy MG, Rutkowski P, et al. Activity and safety of crizotinib in patients with alveolar soft part sarcoma with rearrangement of TFE3: European Organization for Research and Treatment of Cancer (EORTC) phase II trial 90101 ‘CREATE’. Ann Oncol. 2018;29(3):758–65.
Schoffski P, Wozniak A, Stacchiotti S, Rutkowski P, Blay JY, Lindner LH, et al. Activity and safety of crizotinib in patients with advanced clear-cell sarcoma with MET alterations: European Organization for Research and Treatment of Cancer phase II trial 90101 ‘CREATE’. Ann Oncol. 2017;28(12):3000–8.
Schoffski P, Wozniak A, Escudier B, Rutkowski P, Anthoney A, Bauer S, et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur J Cancer (Oxford, England: 1990). 2017;87:147–63.
Ou SI, Govindan R, Eaton KD, Otterson GA, Gutierrez ME, Mita AC, et al. Phase I results from a study of crizotinib in combination with erlotinib in patients with advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2017;12(1):145–51.
Janne PA, Shaw AT, Camidge DR, Giaccone G, Shreeve SM, Tang Y, et al. Combined pan-HER and ALK/ROS1/MET inhibition with dacomitinib and crizotinib in advanced non-small cell lung cancer: results of a phase I study. J Thorac Oncol. 2016;11(5):737–47.
Kato S, Jardim DL, Johnson FM, Subbiah V, Piha-Paul S, Tsimberidou AM, et al. Phase I study of the combination of crizotinib (as a MET inhibitor) and dasatinib (as a c-SRC inhibitor) in patients with advanced cancer. Invest New Drugs. 2018;36(3):416–23.
Broniscer A, Jia S, Mandrell B, Hamideh D, Huang J, Onar-Thomas A, et al. Phase 1 trial, pharmacokinetics, and pharmacodynamics of dasatinib combined with crizotinib in children with recurrent or progressive high-grade and diffuse intrinsic pontine glioma. Pediatr Blood Cancer. 2018;65(7):e27035.
Spigel DR, Reynolds C, Waterhouse D, Garon EB, Chandler J, Babu S, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation—positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol. 2018;13(5):682–8.
Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308.
Bentzien F, Zuzow M, Heald N, Gibson A, Shi Y, Goon L, et al. In vitro and in vivo activity of cabozantinib (XL184), an inhibitor of RET, MET, and VEGFR2, in a model of medullary thyroid cancer. Thyroid. 2013;23(12):1569–77.
Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–6.
Choueiri TK, Pal SK, McDermott DF, Morrissey S, Ferguson KC, Holland J, et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol. 2014;25(8):1603–8.
Nguyen HM, Ruppender N, Zhang X, Brown LG, Gross TS, Morrissey C, et al. Cabozantinib inhibits growth of androgen-sensitive and castration-resistant prostate cancer and affects bone remodeling. PLoS One. 2013;8(10):e78881.
Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1089–96.
Drilon A, Rekhtman N, Arcila M, Wang L, Ni A, Albano M, et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653–60.
Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–46.
Cabanillas ME, Brose MS, Holland J, Ferguson KC, Sherman SI. A phase I study of cabozantinib (XL184) in patients with differentiated thyroid cancer. Thyroid. 2014;24(10):1508–14.
Cabanillas ME, de Souza JA, Geyer S, Wirth LJ, Menefee ME, Liu SV, et al. Cabozantinib as salvage therapy for patients with tyrosine kinase inhibitor-refractory differentiated thyroid cancer: results of a multicenter phase II International Thyroid Oncology Group trial. J Clin Oncol. 2017;35(29):3315–21.
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23.
Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591–7.
Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–27.
Motzer RJ, Escudier B, Powles T, Scheffold C, Choueiri TK. Long-term follow-up of overall survival for cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. 2018;118(9):1176–8.
Choueiri TK, Halabi S, Sanford B, Hahn O, Michaelson MD, Walsh M, et al. CABOzantinib versus SUNitinib (CABOSUN) as initial targeted therapy for patients with metastatic renal cell carcinoma (mRCC) of poor and intermediate risk groups: results from ALLIANCE A031203 trial. Ann Oncol. 2016;27(suppl_6):LBA30_PR-LBA_PR.
Abou-Alfa GK, Meyer T, Cheng A-L, El-Khoueiry AB, Rimassa L, Ryoo B-Y, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63.
Nadal R, Mortazavi A, Stein M, Pal SK, Davarpanah N, Parnes HL, et al. 846OFinal results of a phase I study of cabozantinib (cabo) plus nivolumab (nivo) and cabonivo plus ipilimumab (Ipi) in patients (pts) with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) malignancies. Ann Oncol. 2017;28(suppl_5):mdx371.001–mdx371.001.
Nadal R, Mortazavi A, Stein MN, Pal SK, Lee DK, Parnes HL, et al. Clinical efficacy of cabozantinib plus nivolumab (CaboNivo) and CaboNivo plus ipilimumab (CaboNivoIpi) in patients (pts) with chemotherapy-refractory metastatic urothelial carcinoma (mUC) either naïve (n) or refractory (r) to checkpoint inhibitor (CPI). J Clin Oncol. 2018;36(15_suppl):4528.
Choueiri TK, Apolo AB, Powles T, Escudier B, Aren OR, Shah A, et al. A phase 3, randomized, open-label study of nivolumab combined with cabozantinib vs sunitinib in patients with previously untreated advanced or metastatic renal cell carcinoma (RCC; CheckMate 9ER). J Clin Oncol. 2018;36(15_suppl):TPS4598-TPS.
Tolaney SM, Nechushtan H, Ron IG, Schoffski P, Awada A, Yasenchak CA, et al. Cabozantinib for metastatic breast carcinoma: results of a phase II placebo-controlled randomized discontinuation study. Breast Cancer Res Treat. 2016;160(2):305–12.
Hirota T, Muraki S, Ieiri I. Clinical pharmacokinetics of anaplastic lymphoma kinase inhibitors in non-small-cell lung cancer. Clin Pharmacokinet. 2018. https://doi.org/10.1007/s40262-018-0689-7.
Tan W, Wilner KD, Bang Y, Kwak EL, Maki RG, Camidge DR, et al. Pharmacokinetics (PK) of PF-02341066, a dual ALK/MET inhibitor after multiple oral doses to advanced cancer patients. J Clin Oncol. 2010;28(15_suppl):2596.
Xu H, O’Gorman M, Boutros T, Brega N, Kantaridis C, Tan W, et al. Evaluation of crizotinib absolute bioavailability, the bioequivalence of three oral formulations, and the effect of food on crizotinib pharmacokinetics in healthy subjects. J Clin Pharmacol. 2015;55(1):104–13.
Johnson TR, Tan W, Goulet L, Smith EB, Yamazaki S, Walker GS, et al. Metabolism, excretion and pharmacokinetics of [14C]crizotinib following oral administration to healthy subjects. Xenobiotica. 2015;45(1):45–59.
El-Khoueiry AB, Sarantopoulos J, O’Bryant CL, Ciombor KK, Xu H, O’Gorman M, et al. Evaluation of hepatic impairment on pharmacokinetics and safety of crizotinib in patients with advanced cancer. Cancer Chemother Pharmacol. 2018;81(4):659–70.
Tan W, Yamazaki S, Johnson TR, Wang R, O’Gorman MT, Kirkovsky L, et al. Effects of renal function on crizotinib pharmacokinetics: dose recommendations for patients with ALK-positive non-small cell lung cancer. Clin Drug Investig. 2017;37(4):363–73.
Shi J, Montay G, Chapel S, Hardy P, Barrett JS, Sack M, et al. Pharmacokinetics and safety of the ketolide telithromycin in patients with renal impairment. J Clin Pharmacol. 2004;44(3):234–44.
Zhou WJ, Zhang X, Cheng C, Wang F, Wang XK, Liang YJ, et al. Crizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoprotein. Br J Pharmacol. 2012;166(5):1669–83.
Eliesen GAM, van den Broek P, van den Heuvel JJ, Bilos A, Pertijs J, van Drongelen J, et al. Editor’s highlight: placental disposition and effects of crizotinib: an ex vivo study in the isolated dual-side perfused human cotyledon. Toxicol Sci. 2017;157(2):500–9.
Tang SC, Nguyen LN, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer. 2014;134(6):1484–94.
Sato T, Ito H, Hirata A, Abe T, Mano N, Yamaguchi H. Interactions of crizotinib and gefitinib with organic anion-transporting polypeptides (OATP)1B1, OATP1B3 and OATP2B1: gefitinib shows contradictory interaction with OATP1B3. Xenobiotica. 2018;48(1):73–8.
Wang E, Nickens DJ, Bello A, Khosravan R, Amantea M, Wilner KD, et al. Clinical implications of the pharmacokinetics of crizotinib in populations of patients with non-small cell lung cancer. Clin Cancer Res. 2016;22(23):5722–8.
Lacy S, Yang B, Nielsen J, Miles D, Nguyen L, Hutmacher M. A population pharmacokinetic model of cabozantinib in healthy volunteers and patients with various cancer types. Cancer Chemother Pharmacol. 2018;81(6):1071–82.
Lacy SA, Miles DR, Nguyen LT. Clinical pharmacokinetics and pharmacodynamics of cabozantinib. Clin Pharmacokinet. 2017;56(5):477–91.
Nguyen L, Benrimoh N, Xie Y, Offman E, Lacy S. Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adults. Anticancer Drugs. 2016;27(7):669–78.
Bersanelli M, Buti S. Cabozantinib in metastatic renal cell carcinoma: latest findings and clinical potential. Ther Adv Med Oncol. 2017;9(10):627–36.
Foti RS, Rock DA, Wienkers LC, Wahlstrom JL. Selection of alternative CYP3A4 probe substrates for clinical drug interaction studies using in vitro data and in vivo simulation. Drug Metab Dispos. 2010;38(6):981–7.
Lacy S, Hsu B, Miles D, Aftab D, Wang R, Nguyen L. Metabolism and disposition of cabozantinib in healthy male volunteers and pharmacologic characterization of its major metabolites. Drug Metab Dispos. 2015;43(8):1190–207.
Sharma N, Adjei AA. In the clinic: ongoing clinical trials evaluating c-MET-inhibiting drugs. Ther Adv Med Oncol. 2011;3(1 Suppl):S37–50.
Wakelee HA, Gettinger S, Engelman J, Jänne PA, West H, Subramaniam DS, et al. A phase Ib/II study of cabozantinib (XL184) with or without erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2017;79(5):923–32.
Crizotinib (Xalkori®) DDI: http://www.pdr.net/drug-summary/Xalkori-crizotinib-472. Accessed 20 Sept 2018.
Cabozantinib (Cabometyx®) DDI: http://www.pdr.net/drug-summary/Cabometyx-cabozantinib-3908. Accessed 20 Sept 2018.
Nickens D, Tan W, Wilner K, Camidge DR, Shapiro G, Dezube B, et al. Abstract 1673: A pharmacokinetics/pharmacodynamics evaluation of the concentration-QTc relationship of PF-02341066 (PF-1066), an ALK and c-MET/HGFR dual inhibitor administered orally to patients with advanced cancer. Cancer Res. 2010;70(8 Supplement):1673.
Oser MG, Jänne PA. A severe photosensitivity dermatitis caused by crizotinib. J Thoracic Oncol. 2014;9(7):e51–3.
Yang S, Wu L, Li X, Huang J, Zhong J, Chen X. Crizotinib-associated toxic epidermal necrolysis in an ALK-positive advanced NSCLC patient. Mol Clin Oncol. 2018;8(3):457–9.
FAERS Public Dashboard: https://fis.fda.gov/sense/app/d10be6bb-494e-4cd2-82e4-0135608ddc13/sheet/45beeb74-30ab-46be-8267-5756582633b4/state/analysis. Accessed 10 Oct 2018.
Crizotinib (Xalkori®) EPAS: https://www.ema.europa.eu/documents/procedural-steps-after/xalkori-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf. Accessed 8 Oct 2018.
Cabozantinib (Cometriq®) EPAS: https://www.ema.europa.eu/documents/procedural-steps-after/cometriq-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf. Accessed 8 Oct 2018.
Cabozantinib (Cabometyx®) EPAS: https://www.ema.europa.eu/documents/procedural-steps-after/cabometyx-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf. Accessed 8 Oct 2018.
Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(11):1467–82.
Doi T, Kang Y-K, Muro K, Jiang Y, Jain RK, Lizambri R. A phase 3, multicenter, randomized, double-blind, placebo-controlled study of rilotumumab in combination with cisplatin and capecitabine (CX) as first-line therapy for Asian patients (pts) with advanced MET-positive gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The RILOMET-2 trial. J Clin Oncol. 2015;33(3_suppl):TPS226-TPS.
Van Cutsem E, Eng C, Nowara E, Swieboda-Sadlej A, Tebbutt NC, Mitchell E, et al. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin Cancer Res. 2014;20(16):4240–50.
Affronti ML, Jackman JG, McSherry F, Herndon JE, Massey EC, Lipp E, et al. Phase II study to evaluate the efficacy and safety of rilotumumab and bevacizumab in subjects with recurrent malignant glioma. Oncologist. 2018;23(8):889–98.
Tarhini AA, Rafique I, Floros T, Tran P, Gooding WE, Villaruz LC, et al. Phase 1/2 study of rilotumumab (AMG 102), a hepatocyte growth factor inhibitor, and erlotinib in patients with advanced non-small cell lung cancer. Cancer. 2017;123(15):2936–44.
Mok T, Park K, Geater S, Agarwal S, Han M, Komarnitsky P, et al. A randomized phase 2 study with exploratory biomarker analysis of ficlatuzumab, a humanized hepatocyte growth factor (HGF) inhibitory monoclonal antibody, in combination with gefitinib versus gefitinib alone in Asian patients with lung adenocarcinoma. Ann Oncol. 2012;23(Suppl 9):1198P.
Mok TS, Geater SL, Su WC, Tan EH, Yang JC, Chang GC, et al. A randomized phase 2 study comparing the combination of ficlatuzumab and gefitinib with gefitinib alone in Asian patients with advanced stage pulmonary adenocarcinoma. J Thorac Oncol. 2016;11(10):1736–44.
Spigel DR, Edelman MJ, O’Byrne K, Paz-Ares L, Mocci S, Phan S, et al. Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-small-cell lung cancer: METLung. J Clin Oncol. 2017;35(4):412–20.
Bendell JC, Hochster HS, Hart LL, Firdaus I, Mace JR, McFarlane JJ, et al. A randomized, double-blind, phase II study of first-line FOLFOX plus bevacizumab with onartuzumab versus placebo in patients with metastatic colorectal cancer (mCRC). J Clin Oncol. 2015;33(3_suppl):663.
Shah MA, Bang Y-J, Lordick F, Alsina M, Chen M, Hack SP, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3(5):620–7.
Rosen LS, Goldman JW, Algazi AP, Turner PK, Moser B, Hu T, et al. A first-in-human phase I study of a bivalent MET antibody, emibetuzumab (LY2875358), as monotherapy and in combination with erlotinib in advanced cancer. Clin Cancer Res. 2017;23(8):1910–9.
Hultberg A, Morello V, Huyghe L, De Jonge N, Blanchetot C, Hanssens V, et al. Depleting MET-expressing tumor cells by ADCC provides a therapeutic advantage over inhibiting HGF/MET signaling. Can Res. 2015;75(16):3373–83.
Aftimos PG, Barthelemy P, Rolfo CD, Hanssens V, Jonge ND, Silence K, et al. A phase I, first-in-human study of argx-111, a monoclonal antibody targeting c-met in patients with solid tumors. J Clin Oncol. 2015;33(15_suppl):2580.
Kang Y-K, LoRusso P, Salgia R, Yen C-J, Lin C-C, Ramanathan RK, et al. Phase I study of ABT-700, an anti-c-Met antibody, in patients (pts) with advanced gastric or esophageal cancer (GEC). J Clin Oncol. 2015;33(3_suppl):167.
Strickler JH, Weekes CD, Nemunaitis J, Ramanathan RK, Heist RS, Morgensztern D, et al. First-in-human phase I, dose-escalation and -expansion study of telisotuzumab vedotin, an antibody-drug conjugate targeting c-Met, in Patients with advanced solid tumors. J Clin Oncol. 2018:Jco2018787697.
Goldman J, Angevin E, Strickler J, Camidge D, Heist R, Morgensztern D, et al. MA 02.10 Phase I study of ABBV-399 (telisotuzumab vedotin) as monotherapy and in combination with erlotinib in NSCLC. J Thorac Oncol. 2017;12(11):S1805-S6.
Schuler MH, Berardi R, Lim W-T, Geel RV, De Jonge MJ, Bauer TM, et al. Phase (Ph) I study of the safety and efficacy of the cMET inhibitor capmatinib (INC280) in patients (pts) with advanced cMET + non-small cell lung cancer (NSCLC). J Clin Oncol. 2016;34(15-suppl):9067.
Wu Y-L, Kim D-W, Felip E, Zhang L, Liu X, Zhou CC, et al. Phase (Ph) II safety and efficacy results of a single-arm ph ib/II study of capmatinib (INC280) + gefitinib in patients (pts) with EGFR-mutated (mut), cMET-positive (cMET +) non-small cell lung cancer (NSCLC). J Clin Oncol. 2016;34(15-suppl):9020.
Felip E, Horn L, Patel JD, Sakai H, Scheele J, Bruns R, et al. Tepotinib in patients with advanced non-small cell lung cancer (NSCLC) harboring MET exon 14-skipping mutations: phase II trial. J Clin Oncol. 2018;36(15_suppl):9016.
Qin S, Kim T-Y, Lim HY, Ryoo B-Y, Scheele J, Zhou D, et al. Phase Ib trial of tepotinib in Asian patients with advanced hepatocellular carcinoma (HCC): final data including long-term outcomes. J Clin Oncol. 2017;35(15_suppl):4087.
Yasui H, Go N, Yang H, Amore BM, Jung AS, Doi T. A Phase 1 study evaluating AMG 337 in Asian patients with advanced solid tumors. Jpn J Clin Oncol. 2017;47(8):772–6.
Chen HM, Tsai CH, Hung WC. Foretinib inhibits angiogenesis, lymphangiogenesis and tumor growth of pancreatic cancer in vivo by decreasing VEGFR-2/3 and TIE-2 signaling. Oncotarget. 2015;6(17):14940–52.
Yau TCC, Lencioni R, Sukeepaisarnjaroen W, Chao Y, Yen CJ, Lausoontornsiri W, et al. A phase I/II multicenter study of single-agent foretinib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2017;23(10):2405–13.
Eder JP, Shapiro GI, Appleman LJ, Zhu AX, Miles D, Keer H, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16(13):3507–16.
Shapiro GI, McCallum S, Adams LM, Sherman L, Weller S, Swann S, et al. A phase 1 dose-escalation study of the safety and pharmacokinetics of once-daily oral foretinib, a multi-kinase inhibitor, in patients with solid tumors. Invest New Drugs. 2013;31(3):742–50.
Leighl NB, Tsao M-S, Liu G, Tu D, Ho C, Shepherd FA, et al. A phase I study of foretinib plus erlotinib in patients with previously treated advanced non-small cell lung cancer: Canadian cancer trials group IND.196. Oncotarget. 2017;8(41):69651–62.
Yan SB, Peek VL, Ajamie R, Buchanan SG, Graff JR, Heidler SA, et al. LY2801653 is an orally bioavailable multi-kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti-tumor activities in mouse xenograft models. Invest New Drugs. 2013;31(4):833–44.
Konicek BW, Capen AR, Credille KM, Ebert PJ, Falcon BL, Heady GL, et al. Merestinib (LY2801653) inhibits neurotrophic receptor kinase (NTRK) and suppresses growth of NTRK fusion bearing tumors. Oncotarget. 2018;9(17):13796–806.
Fujita H, Gomori A, Fujioka Y, Kataoka Y, Tanaka K, Hashimoto A, et al. High potency VEGFRs/MET/FMS triple blockade by TAS-115 concomitantly suppresses tumor progression and bone destruction in tumor-induced bone disease model with lung carcinoma cells. PLoS One. 2016;11(10):e0164830.
Yamada S, Imura Y, Nakai T, Nakai S, Yasuda N, Kaneko K, et al. Therapeutic potential of TAS-115 via c-MET and PDGFRα signal inhibition for synovial sarcoma. BMC Cancer. 2017;17:334.
Matsubara N, Naito Y, Sasaki M, Yamamoto N, Takahashi S, Uemura H. 796P - Phase I expansion cohort of TAS-115, a novel oral MET/VEGFR/FMS inhibitor, for castration-resistant prostate cancer patients (CRPC pts) with bone metastases. Ann Oncol. 2017;28(suppl_5):v269–94.
Rodon J, Postel-Vinay S, Hollebecque A, Nuciforo P, Azaro A, Cattan V, et al. First-in-human phase I study of oral S49076, a unique MET/AXL/FGFR inhibitor, in advanced solid tumours. Eur J Cancer (Oxford, England: 1990). 2017;81:142–50.
Chang G, Curigliano G, Lim W, Viteri S, Ciardiello F, Hida T, et al. MA 12.02 Phase I/II study of S49076, a MET/AXL/FGFR inhibitor, combined with gefitinib in NSCLC patients progressing on EGFR TKI. Journal of Thoracic Oncology. 2017;12(11):S1847-S8.
Mughal A, Aslam HM, Sheikh A, Khan AMH, Saleem S. c-Met inhibitors. Infect Agents Cancer. 2013;8:13.
Michieli P, Di Nicolantonio F. Targeted therapies: tivantinib–a cytotoxic drug in MET inhibitor’s clothes? Nat Rev Clin Oncol. 2013;10(7):372–4.
Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14(1):55–63.
Rimassa L, Assenat E, Peck-Radosavljevic M, Pracht M, Zagonel V, Mathurin P, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19(5):682–93.
Scagliotti G, von Pawel J, Novello S, Ramlau R, Favaretto A, Barlesi F, et al. Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(24):2667–74.
Scagliotti GV, Shuster D, Orlov S, von Pawel J, Shepherd FA, Ross JS, et al. Tivantinib in combination with erlotinib versus erlotinib alone for EGFR-mutant NSCLC: an exploratory analysis of the phase 3 MARQUEE study. J Thorac Oncol. 2018;13(6):849–54.
Finisguerra V, Prenen H, Mazzone M. Preclinical and clinical evaluation of MET functions in cancer cells and in the tumor stroma. Oncogene. 2016;35(42):5457–67.
Author information
Authors and Affiliations
Contributions
FB, AP and NIM-R drafted the manuscript. FB supervised the manuscript. All authors directly provided their contribution, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Francesca Battaglin has received honoraria for lectures from Eli Lilly and Company, and travel/accommodation support from Bayer and Amgen Inc. Heinz-Josef Lenz has received clinical trial financial support from Merck Serono and Roche, honoraria for advisory board membership and lectures from Bayer, Boehringer Ingelheim, Genentech, Pfizer, Merck Serono and Roche, and travel/accommodation support from Bayer, Merck Serono and Roche. Fotios Loupakis has received travel/accommodation support from Amgen Inc., Merck Serono and Roche. Sara Lonardi has received research funding from Merck Serono and Amgen Inc., honoraria for consulting/advisory roles from Amgen Inc., Bayer Healthcare, Merck Serono and Eli Lilly and Company, and honoraria for speakers’ bureau from Eli Lilly and Company, Bristol-Myers Squibb and Roche. Alberto Puccini, Nagore I. Marín-Ramos, Francesca Bergamo and Marta Schirripa have no conflicts of interest that are directly relevant to the content of this manuscript.
Funding
This manuscript was partly supported by the National Cancer Institute (grant number P30CA014089), the Gloria Borges WunderGlo Foundation–The Wunder Project, the Dhont Family Foundation, the San Pedro Peninsula Cancer Guild, the Daniel Butler Research Fund, and the Call to Cure Research Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Additional information
Part of a theme issue on “Safety of Novel Anticancer Therapies: Future Perspectives”. Guest Editors: Rashmi R. Shah, Giuseppe Curigliano.
Rights and permissions
About this article
Cite this article
Puccini, A., Marín-Ramos, N.I., Bergamo, F. et al. Safety and Tolerability of c-MET Inhibitors in Cancer. Drug Saf 42, 211–233 (2019). https://doi.org/10.1007/s40264-018-0780-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0780-x