Abstract
Introduction
Although tamsulosin is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH), it has also been assessed in clinical studies for other conditions/symptoms and in other populations such as women and children. In this systematic review of randomized studies, the overall safety of tamsulosin was assessed, focusing on these understudied populations.
Methods
Literature searches were conducted using Embase, Medline, and PubMed (inception–December 2015). A study was included if patients were randomized to receive treatment with any dose of tamsulosin capsules, tablets, or an oral controlled absorption system and numerical safety results were reported.
Results
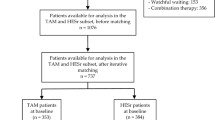
Overall, 160 articles involving 46,072 participants met the inclusion criteria. Of these, four studies included women only and three included children. The mean [standard deviation (SD)] age ranged from 7.3 (4.2) to 76.8 (7.1) years. The studies (n; %) evaluated healthy subjects (18; 11%) or patients with lower urinary tract symptoms/BPH (90; 56%), ureteral stones/renal colic (42; 26%), prostatitis (4; 3%), or other conditions (6; 4%). Patients discontinued tamsulosin primarily because of adverse events (AEs) or insufficient response. AEs in women and children were abdominal pain, asthenia, constipation, dizziness, dry mouth, drowsiness, dyspepsia, headache, incontinence, nasal congestion, nausea, orthostatic hypotension, and somnolence. Due to heterogeneity across studies, statistical analysis could not be conducted.
Discussion
No unexpected AEs were observed in an all-comers population treated with tamsulosin for various conditions/symptoms. The overall safety profile in women and children seemed to be generally consistent with the profile in men, the indicated population.

Similar content being viewed by others
References
American Urological Association Guideline. Management of benign prostatic hyperplasia (BPH). American Urological Association Education and Research, Inc. 2010. https://www.auanet.org/guidelines/benign-prostatic-hyperplasia-(2010-reviewed-and-validity-confirmed-2014). Accessed 21 June 2017.
Flomax prescribing information. http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Flomax%20Caps/Flomax.pdf. Accessed 21 June 2017.
Campschroer T, Zhu Y, Duijvesz D, Grobbee DE, Lock MT. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014;(4):CD008509. https://doi.org/10.1002/14651858.cd008509.pub2.
Chughtai B, Forde JC, Thomas DD, Laor L, Hossack T, Woo HH, et al. Benign prostatic hyperplasia. Nat Rev Dis Primers. 2016;2:16031. https://doi.org/10.1038/nrdp.2016.31.
Schulman CC. Tamsulosin modified release and oral controlled absorption system in the management of lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Expert Opin Drug Metab Toxicol. 2008;4(6):771–82. https://doi.org/10.1517/17425255.4.6.771.
Yuan J, Liu Y, Yang Z, Qin X, Yang K, Mao C. The efficacy and safety of alpha-1 blockers for benign prostatic hyperplasia: an overview of 15 systematic reviews. Curr Med Res Opin. 2013;29(3):279–87. https://doi.org/10.1185/03007995.2013.766594.
Malo C, Audette-Côté JS, Emond M, Turgeon AF. Tamsulosin for treatment of unilateral distal ureterolithiasis: a systematic review and meta-analysis. CJEM. 2014;16(3):229–42.
Velázquez N, Zapata D, Wang HH, Wiener JS, Lipkin ME, Routh JC. Medical expulsive therapy for pediatric urolithiasis: systematic review and meta-analysis. J Pediatr Urol. 2015;11(6):321–7. https://doi.org/10.1016/j.jpurol.2015.04.036.
Fisher E, Subramonian K, Omar MI. The role of alpha blockers prior to removal of urethral catheter for acute urinary retention in men. Cochrane Database Syst Rev. 2014;(6):CD006744. https://doi.org/10.1002/14651858.cd006744.pub3.
Nickel JC. Role of alpha1-blockers in chronic prostatitis syndromes. BJU Int. 2008;101(Suppl 3):11–6. https://doi.org/10.1111/j.1464-410X.2008.07496.x.
Boyd K, Hilas O. Alpha-adrenergic blockers for the treatment of lower-urinary-tract symptoms and dysfunction in women. Ann Pharmacother. 2014;48(6):711–22. https://doi.org/10.1177/1060028014524174.
Lee KS, Han DH, Lee YS, Choo MS, Yoo TK, Park HJ, et al. Efficacy and safety of tamsulosin for the treatment of non-neurogenic voiding dysfunction in females: a 8-week prospective study. J Korean Med Sci. 2010;25(1):117–22. https://doi.org/10.3346/jkms.2010.25.1.117.
Meyer LE, Brown JN. Tamsulosin for voiding dysfunction in women. Int Urol Nephrol. 2012;44(6):1649–56. https://doi.org/10.1007/s11255-012-0275-0.
Tasian GE, Cost NG, Granberg CF, Pulido JE, Rivera M, Schwen Z, et al. Tamsulosin and spontaneous passage of ureteral stones in children: a multi-institutional cohort study. J Urol. 2014;192(2):506–11. https://doi.org/10.1016/j.juro.2014.01.091.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. https://doi.org/10.1016/j.ijsu.2010.02.007.
OCEBM Levels of Evidence Working Group. “The Oxford 2011 Levels of Evidence”. Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653. Accessed 10 Mar 2018.
Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 updated June 2010. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 21 June 2017.
Hajebrahimi S, Asrbadr YA, Azaripour A, Sadeghi-Bazargani H. Effect of tamsulosin versus prazosin on clinical and urodynamic parameters in women with voiding difficulty: a randomized clinical trial. Int J Gen Med. 2011;4:35–9. https://doi.org/10.2147/IJGM.S16063.
Kim SO, Hwang EC, Oh KJ, Kwon D, Park K, Ryu SB. Efficacy and safety of combined therapy with tamsulosin and tolterodine in female patients with a maximal flow rate less than 12 ml/s. Int Urogynecol J. 2011;22(10):1287–91. https://doi.org/10.1007/s00192-011-1453-9.
Pummangura N, Kochakarn W. Efficacy of tamsulosin in the treatment of lower urinary tract symptoms (LUTS) in women. Asian J Surg. 2007;30(2):131–7.
Robinson D, Cardozo L, Terpstra G, Bolodeoku J. Tamsulosin Study Group. A randomized double-blind placebo-controlled multicentre study to explore the efficacy and safety of tamsulosin and tolterodine in women with overactive bladder syndrome. BJU Int. 2007;100(4):840–5.
Aldaqadossi HA, Shaker H, Saifelnasr M, Gaber M. Efficacy and safety of tamsulosin as a medical expulsive therapy for stones in children. Arab J Urol. 2015;13(2):107–11. https://doi.org/10.1016/j.aju.2015.02.007.
Mokhless I, Zahran AR, Youssif M, Fahmy A. Tamsulosin for the management of distal ureteral stones in children: a prospective randomized study. J Pediatr Urol. 2012;8(5):544–8. https://doi.org/10.1016/j.jpurol.2011.09.008.
Homsy Y, Arnold P, Zhang W. Phase IIb/III dose ranging study of tamsulosin as treatment for children with neuropathic bladder. J Urol. 2011;186(5):2033–9. https://doi.org/10.1016/j.juro.2011.07.021.
Wilt TJ, MacDonald R, Nelson D. Tamsulosin for treating lower urinary tract symptoms compatible with benign prostatic obstruction: a systematic review of efficacy and adverse effects. J Urol. 2002;167(1):177–83.
Nickel JC, Sander S, Moon TD. A meta-analysis of the vascular-related safety profile and efficacy of alpha-adrenergic blockers for symptoms related to benign prostatic hyperplasia. Int J Clin Pract. 2008;62(10):1547–59. https://doi.org/10.1111/j.1742-1241.2008.01880.x.
Karesh K. Pediatric focused safety review: Flomax® (tamsulosin hydrochloride). U. S. Food and Drug Administration 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM289947.pdf. Accessed 18 October 2016.
Bailey G, Vaughan L, Rose C, Krambeck A. Perinatal outcomes with tamsulosin therapy for symptomatic urolithiasis. J Urol. 2016;195(1):99–103. https://doi.org/10.1016/j.juro.2015.06.097.
Acknowledgments
Writing, editorial support, and formatting assistance was provided by Suchita Nath-Sain, PhD, and Maribeth Bogush, PhD, of Cactus Communications, which was contracted and compensated by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) for these services. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. Steven A. Kaplan and Bilal I. Chughtai have no conflicts of interest to declare.
Funding
The study was funded by Boehringer Ingelheim Pharmaceuticals, Inc.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaplan, S.A., Chughtai, B.I. Safety of Tamsulosin: A Systematic Review of Randomized Trials with a Focus on Women and Children. Drug Saf 41, 835–842 (2018). https://doi.org/10.1007/s40264-018-0674-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0674-y