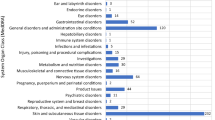
Abstract
Biosimilars are biological medicines, the active substances of which are highly similar to those of biologics that have already been authorized. As for any other medicine, the applicant of the biosimilar marketing authorization must submit a risk-management plan (RMP)/pharmacovigilance plan. The pharmacovigilance plan should take into account risks identified during product development, the potential risks and how those risks will be addressed after authorization of the product.
Recently, new European Pharmacovigilance legislation has been implemented, ensuring proper risk management through the recording of suspected adverse drug reactions and data collection from all stakeholders. The new regulation entails a reduction of the administrative burden on companies and regulatory agencies, as obligations of the responsible parties are clearly established and duplication of effort avoided.
This article analyzes the new European Pharmacovigilance System requirements, with special focus on those medicines requiring additional monitoring, such as biosimilars, which are priorities for pharmacovigilance. Further, it provides the new obligations to marketing authorization holders, such as the continuous benefit–risk assessment.


Similar content being viewed by others
References
Regulation (EU) No 1235/2010 of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, and Regulation (EC) No 1394/2007 on advanced therapy medicinal products. Official Journal of the European Union L 348/1, 31/12/2010.
Directive 2010/84/EU of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance, Directive 2001/83/EC on the Community code relating to medicinal products for human use. Official Journal of the European Union L 348/74, 31/12/2010.
EudraLex [Homepage]. Volume 9A guidelines on pharmacovigilance for medicinal products for human use. In: The rules governing medicinal products in the European Union. 2007. http://ec.europa.eu. Accessed 1 Jul 2013.
EMA/838713/2011. Guideline on good pharmacovigilance practices (GVP). Module V: risk management systems. 2012. http://www.ema.europa.eu. Accessed 1 Jul 2013.
Amendments to the pharmacovigilance legislation: new notification requirements for marketing-authorisation holders. 2013. http://www.ema.europa.eu. Accessed 1 Jul 2013.
Zuñiga L, Calvo B. Biosimilars: pharmacovigilance and risk management. Pharmacoepidem Drug Saf. 2010;19:661–9.
Casadevall N, Edwards IR, Felix T, Graze PR, Litten JB, Strober BE, Warnock DG. Pharmacovigilance and biosimilars: considerations, needs and challenges. Expert Opin Biol Ther. 2013;13:1039–47.
EMA/144772/2013. Patient Health Protection. Implementation plan for the introduction of the new pharmacovigilance legislation requirements into the product information of centrally approved medicinal products. 2013. http://www.ema.europa.eu. Accessed 1 Jul 2013.
Borg JJ, Aislaitner G, Pirozynski M, Mifsud S. Strengthening and rationalizing pharmacovigilance in the EU: where is Europe heading to? A review of the new EU legislation on pharmacovigilance. Drug Saf. 2011;34:187–97.
International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH E2E: Pharmacovigilance Planning. 2004. http://www.ich.org. Accessed 1 Jul 2013.
Pharmacovigilance Focus. Biosimilar medicines and safety: new challenges for pharmacovigilance. WHO Drug Inf. 2009; 23:87–94. http://www.who.int/druginformation. Accessed 1 Jul 2013.
Berns JS, Elzein H, Lynn RI, Fishbane S, Meisels IS, Deoreo PB. Hemoglobin variability in epoetin-treated hemodialysis patients. Kidney Int. 2003;64:1514–21.
Yang W, Israni RK, Brunelli SM, Joffe MM, Fishbane SC, Feldman HI. Hemoglobin variability and mortality in ESRD. J Am Soc Nephrol. 2007;18:3164–70.
Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P, Michaud P, Papo T, Ugo V, Teyssandier I, Varet B, Mayeux P. Pure red-cell aplasia and anti-erythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med. 2002;346:469–75.
Haag-Weber M, Eckardt KU, Hörl WH, Roger SD, Vetter A, Roth K. Safety, immunogenicity and efficacy of subcutaneous biosimilar epoetin-alpha (HX575) in non-dialysis patients with renal anemia: a multi-center, randomized, double-blind study. Clin Nephrol. 2012;77:8–17.
Seidl A, Hainzl O, Richter M, Fischer R, Böhm S, Deutel B, Hartinger M, Windisch J, Casadevall N, London GM, Macdougall I. Tungsten-induced denaturation and aggregation of epoetin alfa during primary packaging as a cause of immunogenicity. Pharm Res. 2012;29:1454–67.
Schellekens H. The first biosimilar epoetin: but how similar is it? Clin J Am Soc Nephrol. 2008;3:174–8.
Bennett CL, Luminari S, Nissenson AR, Tallman MS, Klinge SA, McWilliams N, McKoy JM, Kim B, Lyons EA, Trifilio SM, Raisch DW, Evens AM, Kuzel TM, Schumock GT, Belknap SM, Locatelli F, Rossert J, Casadevall N. Pure red-cell aplasia and epoetin therapy. N Eng J Med. 2004;351:1403–8.
Schellekens H, Jiskoot W. Eprex-associated pure red cell aplasia and leachates. Nat Biotechnol. 2006;24:613–4.
Acknowledgments
No sources of funding were used to assist in the preparation of this article. Begoña Calvo and Leyre Zuñiga have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calvo, B., Zuñiga, L. EU’s New Pharmacovigilance Legislation: Considerations for Biosimilars. Drug Saf 37, 9–18 (2014). https://doi.org/10.1007/s40264-013-0121-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-013-0121-z