Abstract
Background
The antiepileptic drug vigabatrin is associated with characteristic visual field loss (VAVFL) and thinning of the peripapillary retinal nerve fibre layer (PPRNFL); however, the relationship is equivocal.
Objective
The aim of this study was to determine the function–structure relationship associated with long-term exposure to vigabatrin, thereby improving the risk/benefit analysis of the drug.
Methods
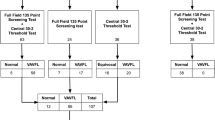
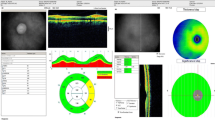
A cross-sectional observational design identified 40 adults who had received long-term vigabatrin for refractory seizures, who had no evidence of co-existing retino-geniculo-cortical visual pathway abnormality, and who had undergone a standardized protocol of perimetry and of optical coherence tomography (OCT) of the PPRNFL. Vigabatrin toxicity was defined as the presence of VAVFL. The function–structure relationship for the superior and inferior retinal quadrants was evaluated by two established models applicable to other optic neuropathies.
Results
The function–structure relationship for each model was consistent with an optic neuropathy. PPRNFL thinning, expressed in micrometres, asymptoted at an equivalent visual field loss of worse than approximately − 10.0 dB, thereby preventing assessment of more substantial thinning. Transformation of the outcomes to retinal ganglion cell soma and axon estimates, respectively, resulted in a linear relationship.
Conclusions
Functional and structural abnormality is strongly related in individuals with vigabatrin toxicity and no evidence of visual pathway comorbidity, thereby implicating retinal ganglion cell dysfunction. OCT affords a limited measurement range compared with perimetry: severity cannot be directly assessed when the PPRNFL quadrant thickness is less than approximately 65 µm, depending on the tomographer. This limitation can be overcome by transformation of thickness to remaining axons, an outcome requiring input from perimetry.




Similar content being viewed by others
References
Mumford JP, Dam M. Meta-analysis of European placebo controlled studies of vigabatrin in drug resistant epilepsy. Br J Clin Pharmacol. 1989;27(Suppl 1):S101–7.
Appleton RE, Peters AC, Mumford JP, Shaw DE. Randomised, placebo controlled study of vigabatrin as first-line treatment of infantile spasms. Epilepsia. 1999;40(11):1627–33.
Krauss G, Faught E, Foroozan R, Pellock JM, Sergott RC, Shields WD, et al. Sabril® registry 5-year results: characteristics of adult patients treated with vigabatrin. Epilepsy Behav. 2016;56(3):15–9.
Pellock JM, Faught E, Foroozan R, Sergott RC, Shields WD, Ziemann A, et al. Which children receive vigabatrin? Characteristics of pediatric patients enrolled in the mandatory FDA registry. Epilepsy Behav. 2016;60(7):174–80.
Eke T, Talbot JF, Lawden MC. Severe persistent visual field constriction associated with vigabatrin. BMJ. 1997;314(7075):180–1.
Miller NR, Johnson MA, Paul SR, Girkin CA, Perry JD, Endres M, et al. Visual dysfunction in patients receiving vigabatrin: clinical and electrophysiologic findings. Neurology. 1999;53(9):2082–7.
Kälviäinen R, Nousiainen I, Mäntyjärvi M, Nikoskelainen E, Partanen J, Partanen K, et al. Vigabatrin, a gabaergic antiepileptic drug, causes concentric visual field defects. Neurology. 1999;53(5):922–6.
Wild JM, Martinez C, Reinshagen G, Harding GF. Characteristics of a unique visual field defect attributed to vigabatrin. Epilepsia. 1999;40(12):1784–94.
Lawden MC, Eke T, Degg C, Harding GF, Wild JM. Visual field defects associated with vigabatrin therapy. J Neurol Neurosurg Psychiatry. 1999;67(6):716–22.
Daneshvar H, Racette L, Coupland SG, Kertes PJ, Guberman A, Zackon D. Symptomatic and asymptomatic visual loss in patients taking vigabatrin. Ophthalmology. 1999;106(9):1792–8.
European Medicines Agency. Opinion of the Committee for proprietary medicinal products pursuant to Article 12 of Council Directive 75/319/EEC as amended for vigabatrin. Annex 1. Scientific conclusions and grounds for amendment of the summaries of product characteristics presented by the EMEA. London: European Medicines Agency; 1999. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Vigabatrin_31/WC500014088.pdf. Accessed 31 July 2018.
Wild JM, Fone DL, Aljarudi S, Lawthom C, Smith PE, Newcombe RG, et al. Modelling the risk of visual field loss arising from long-term exposure to the antiepileptic drug vigabatrin: a cross-sectional approach. CNS Drugs. 2013;27(10):841–9.
Malmgren K, Ben-Menachem E, Frisén L. Vigabatrin visual toxicity: evolution and dose dependence. Epilepsia. 2001;42(5):609–15.
Wild JM, Chiron C, Ahn H, Baulac M, Bursztyn J, Gandolfo E, et al. Visual field loss in patients with refractory partial epilepsy treated with vigabatrin: final results from an open-label, observational, multicenter study. CNS Drugs. 2009;23(11):965–82.
Johnson MA, Krauss GL, Miller NR, Medura M, Paul SR. Visual function loss from vigabatrin: effect of stopping the drug. Neurology. 2000;55(1):40–5.
Nousiainen I, Mäntyjärvi M, Kälviäinen R. No reversion in vigabatrin-associated visual field defects. Neurology. 2001;57(10):1916–7.
Choi HJ, Kim DM. Visual field constriction associated with vigabatrin: retinal nerve fiber photographic correlation. J Neurol Neurosurg Psychiatry. 2004;75(10):1395.
Wild JM, Robson CR, Jones AL, Cunliffe IA, Smith PE. Detecting vigabatrin toxicity by imaging of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2006;47(3):917–24.
Lawthom C, Smith PEM, Wild JM. Nasal retinal nerve fiber layer attenuation: a biomarker for vigabatrin toxicity. Ophthalmology. 2009;116(3):565–71.
Clayton LM, Devile M, Punte T, Kallis C, de Haan GJ, Sander JW, et al. Retinal nerve fiber layer thickness in vigabatrin-exposed patients. Ann Neurol. 2011;69(5):845–54.
Clayton LM, Devile M Punte T de Haan GJ, Sander JW, Acheson JF et al. Patterns of peripapillary retinal nerve fiber layer thinning in vigabatrin-exposed individuals. Ophthalmology 2012;119(10):2152–60.
Origlieri C, Geddie B, Karwoski B, Berl MM, Elling N, McClintock W, et al. Optical coherence tomography to monitor vigabatrin toxicity in children. J AAPOS. 2016;20(2):136–40.
Frisen L, Malmgren K. Characterization of vigabatrin associated optic atrophy. Acta Ophthalmol Scand. 2003;81(5):466–73.
Buncic JR, Westall CA, Panton CM, Munn JR, MacKeen LD, Logan WJ. Characteristic retinal atrophy with secondary “inverse” optic atrophy identifies vigabatrin toxicity in children. Ophthalmology. 2004;111(10):1935–42.
Ravindran J, Blumbergs P, Crompton J, Pietris G, Waddy H. Visual field loss associated with vigabatrin: pathological correlations. J Neurol Neurosurg Psychiatry. 2001;70(6):787–9.
Harding GF, Wild JM, Robertson KA, Rietbrock S, Martinez C. Separating the retinal electrophysiologic effects of vigabatrin. Treatment versus field loss. Neurology. 2000;55(3):347–52.
Wright T, Kumarappah A, Stavropoulos A Reginald A, Buncic JR, Westall CA. Vigabatrin toxicity in infants is associated with retinal defect in adolescence. A prospective observational study. Retina 2017;37(5):858–66.
Sergott RC, Johnson CA, Laxer KD, Wechsler RT, Cherny K, Whittle J, et al. Retinal structure and function in vigabatrin-treated adult patients with refractory complex partial seizures. Epilepsia. 2016;57(10):1634–42.
Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132(3):628–34.
Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135(2):534–41.
Park HY, Park YG, Cho AH, Park CK. Transneuronal retrograde degeneration of the retinal ganglion cells in patients with cerebral infarction. Ophthalmology. 2013;120(6):1292–9.
Mitchell JR, Oliveira C, Tsiouris AJ, Dinkin MJ. Corresponding ganglion cell atrophy in patients with postgeniculate homonymous field loss. J Neuroophthalmol. 2015;35(4):353–9.
Balestrini S, Clayton LM, Bartmann AP, Chinthapalli K, Novy J, Coppola A, et al. Retinal nerve fiber layer thinning is associated with drug resistance in epilepsy. J Neurol Neurosurg Psychiatry. 2016;87(4):396–401.
Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47(5):2006–10.
Mwanza J-C, Budenz DL, Warren JL, Webel AD, Reynolds CE, Barbosa DT, et al. Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br J Ophthalmol. 2015;99(6):732–7.
Mwanza J-C, Kim HY, Budenz DL, Warren JL, Margolis M, Lawrence SD, et al. Residual and dynamic range of retinal nerver fiber layer thickness in glaucoma: comparison of three OCT platforms. Invest Ophtahlmol Vis Sci. 2015;56(11):6344–51.
Price DA, Swanson WH, Horner DG. Using perimetric data to estimate ganglion cell loss for detecting progression of glaucoma: a comparison of models. Ophthalmic Physiol Opt. 2017;37(4):409–19.
Hood DC. Relating nerve fiber layer thickness to behavioural sensitivity in patients with glaucoma. The application of a linear mode. J Opt Soc Am. 2007;24(5):1426–30.
Wheat JL, Rangaswamy NV, Harwerth RS. Correlating RNFL thickness by OCT with perimetric sensitivity in glaucoma patients. J Glaucoma. 2012;21(2):95–101.
Hood DC, Anderson S, Wall M, Kardon RH. Structure versus function in glaucoma: an application of a linear model. Invest Ophthalmol Vis Sci. 2007;48(9):3662–8.
Hood DC, Anderson S, Rouleau J, Wenick AS, Grover LK, Behrens MM, et al. Retinal nerve fiber layer structure versus visual field function in patients with ischemic optic neuropathy. A test of a linear model. Ophthalmology 2008;115(5):904–10.
Cheng H, Laron M, Schiffman JS, Tang RA, Frishman LJ. The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Invest Ophthalmol Vis Sci. 2007;48(12):5798–805.
Sakamento M, Mori S, Akashi A, Inoue Y, Kurimoto T, Kanamori A, et al. Diagnostic utility of combined retinal ganglion cell count estimates in Japanese glaucoma patients. Jpn J Ophthalmol. 2018;62(1):31–40.
Medeiros FA, Lisboa R, Weinreb RN, Girkin CA, Liebmann JM, Zangwill LM. A combined index of structure and function for staging glaucomatous damage. Arch Ophthalmol. 2012;130(9):1107–16.
Medeiros FA, Lisboa R, Weinreb RN, Liebmann JM, Girkin CA, Zangwill LM. Retinal ganglion cell count estimates associated with early development of visual field defects in glaucoma. Ophthalmology. 2013;120(4):736–44.
Zhang C, Tatham AJ, Daga FB, Jammal AA, Medeiros FA. Event-based analysis of visual field change can miss fast glaucoma progression detected by a combined structure and function index. Graefes Arch Clin Exp Ophthalmol. 2018;256(7):1227–34.
Vonthein R, Rauscher S, Paetzold J, Nowomiejska K, Krapp E, Hermann A, et al. The normal age-corrected and reaction time-corrected isopter derived by semi-automated kinetic perimetry. Ophthalmology. 2007;114(6):1065–72.
Bengtsson B, Heijl A. False-negative responses in glaucoma perimetry: indicators of patient performance or test reliability? Invest Ophthalmol Vis Sci. 2000;41(8):2201–4.
Wood JM, Wild JM. Crews SJ. Serial examination of the normal visual field using Octopus automated projection perimetry. Evidence for a learning effect. Acta Ophthalmol (Copenh). 1987;65(3):326–33.
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107(10):1809–15.
Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007;114(5):921–6.
Raza AS, Hood DC. Evaluation of a method for estimating retinal ganglion cell counts using visual fields and optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56(9):2254–68.
Hood DC, Raza AS, Kay KY, Sandler SF, Xin D, Ritch R. A comparison of retinal nerve fiber layer (RNFL) thickness obtained with frequency and time domain optical coherence tomography (OCT). Opt Express. 2009;17(5):3997–4003.
Pierro L, Gagliardi M, Iuliani L, Ambrosi A, Bandello F. Retinal nerve fiber layer thickness reproducibility using seven different OCT combinations. Invest Ophthalmol Vis Sci. 2012;53(9):5912–20.
Chang RT, Knight OJ, Feuer WJ, Budenz DL. Sensitivity and specificity of time-domain versus spectral-domain optical coherence tomography in diagnosing early to moderate glaucoma. Ophthalmology. 2009;116(12):2294–9.
Jeoung JW, Park KH. Comparison of the Cirrus OCT and Stratus OCT on the ability to detect localized retinal nerve fiber layer defects in pre-perimetric glaucoma. Invest Ophthalmol Vis Sci. 2010;51(2):938–45.
Watson GM, Keltner JL, Chin EK, Harvey D, Nguyen A, Park SS. Comparison of retinal nerve fiber layer and central macular thickness measurements among five different optical coherence tomography instruments in patients with multiple sclerosis and optic neuritis. J Neuroophthalmol. 2011;31(2):110–6.
Giambene B, Virgili G, Menchini U. Retinal nerve fiber layer thickness by Stratus and Cirrus OCT in retrobulbar optic neuritis and nonarteritic ischemic optic neuropathy. Eur J Ophthalmol. 2017;27(1):80–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
SA was supported by an unrestricted grant from the Ministry of Higher Education, Kingdom of Saudi Arabia. The latter had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Conflicts of interest
John M. Wild, Saleh Aljarudi, Philip E.M. Smith and Carlo Knupp declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the Local Research and Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study had approval from the Local Research and Ethics Committee. For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Wild, J.M., Aljarudi, S., Smith, P.E.M. et al. The Topographical Relationship between Visual Field Loss and Peripapillary Retinal Nerve Fibre Layer Thinning Arising from Long-Term Exposure to Vigabatrin. CNS Drugs 33, 161–173 (2019). https://doi.org/10.1007/s40263-018-0583-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0583-8