Abstract
Background and objective
Patients with focal seizures recruited into adjunctive antiepileptic drug (AED) trials have become more refractory and severe over time; concurrently, placebo responses have increased. To attempt to account for heterogeneity among trials, propensity-score weighted patient-level data were used to indirectly compare placebo responses reported in brivaracetam and levetiracetam trials.
Methods
Patient-level data from randomised, placebo-controlled brivaracetam (recruited 2007–2014) and levetiracetam (1993–1998) trials were pooled. Consistent inclusion/exclusion criteria were applied and outcomes were defined consistently. Potentially confounding baseline characteristics were adjusted for using propensity score weighting. Weighting success was assessed using placebo response.
Results
In total, 707 and 473 active drug and 399 and 253 placebo patients comprised the brivaracetam and levetiracetam groups, respectively. Before weighting, several baseline variables were significantly different between groups; after weighting, prior vagal nerve stimulation, co-morbid depression and co-morbid anxiety remained different. Before weighting, median seizure frequency reduction was 21.7 and 3.9% in the brivaracetam and levetiracetam placebo arms, respectively; after weighting, median reduction was 15.0 and 6.0%. The comparison of non-randomised groups could be biased by unobserved confounding factors and region of residence. Lifetime AED history was unavailable in the brivaracetam trials and excluded from analysis.
Conclusions
Placebo responses remained different between brivaracetam and levetiracetam trials after propensity score weighting, indicating the presence of residual confounding factors associated with placebo response in these trials. It therefore remains problematic to conduct reliable indirect comparisons of brivaracetam and levetiracetam given the current evidence base, which may apply to comparisons between other AED trials.
Similar content being viewed by others
To attempt to account for heterogeneity among adjunctive antiepileptic drug (AED) trials conducted in different eras, propensity score-weighted patient-level data were used to indirectly compare placebo arms between brivaracetam and levetiracetam clinical trials. |
After weighting, prior vagal nerve stimulation, co-morbid depression and co-morbid anxiety assessed at baseline remained different between groups. Median seizure frequency reduction changed from 21.7 to 15.0% in the brivaracetam placebo arm and from 3.9 to 6.0% in the levetiracetam placebo arm. |
As evidenced by the inability of the propensity score weighting to successfully mitigate placebo response differences, the presence of residual confounding factors associated with placebo response in these AED trials is likely, and indirect comparisons of these two AEDs should be made with caution. |
1 Introduction
Epilepsy is a neurological disorder characterised by abnormal spread of cortical nerve activity resulting in seizures of varying duration and severity [1]. An estimated 50 million people globally are currently affected with epilepsy [2], including 5.1 million people in the USA (in 2013) [3] and 6 million people in Europe (in 2009) [4]. Approximately half of patients diagnosed with epilepsy are successfully treated with an initial antiepileptic drug (AED); the remaining half receive a subsequent AED or adjunctive therapy to address drug intolerance or breakthrough seizures [5]. There are many AEDs available and several pharmacological targets for seizure control, including γ-aminobutyric acid (GABA) receptors, sodium channels, and synaptic vesicle protein 2A (SV2A) [6, 7].
Levetiracetam and brivaracetam are two AEDs that target SV2A to different extents [8]. Levetiracetam is approved for the treatment of focal (partial-onset), myoclonic or tonic-clonic seizures in adults and children; pivotal trial enrolment for levetiracetam as adjunctive therapy took place in the 1990s in the USA and Europe [9]. Brivaracetam is a newer, rationally designed selective SV2A ligand, approximately ten times more potent than levetiracetam. It was recently approved for adjunctive treatment of focal seizures in adults following pivotal clinical trials enrolling patients in the past 10 years. Given that levetiracetam is widely prescribed, and brivaracetam has recently become available, there is clinical interest in comparing their respective efficacies.
There are currently no published head-to-head trials of brivaracetam versus levetiracetam comparing efficacy. In the absence of head-to-head trials, indirect comparisons are frequently used to generate comparative efficacy evidence between AEDs [10,11,12,13,14], most often using the Bucher method [15,16,17,18,19], although the suitability of this approach has some caveats. In many cases, indirect comparisons do not take into account differences in baseline trial or patient characteristics, and assume that all arms within a clinical trial are comparable. However, indirect comparisons should address potential heterogeneity among trials, a particular concern in the case of adjunctive AED clinical trials. One marker of heterogeneity is placebo response rate, which has increased over time in adjunctive AED trials [20]. Reported rates of placebo-treated patients with a ≥50% reduction in focal seizure frequency from baseline (responders) have ranged from 9.9 to 39.0% [21, 22].
There are many potential explanations for this heterogeneity, including the expansion of adjunctive AED trials to more diverse geographic regions, evolving or heterogeneous trial design and analysis, and the availability of a wider range of AEDs. Patients in recent trials of AEDs tried and failed to respond to more AEDs than patients in trials conducted in the 1990s, which may indicate that patients enrolled in recent trials are more refractory, or may simply reflect the greater number of available AEDs in recent years. Additionally, there may be differences in clinical characteristics of patients, such as burden of co-morbidities or epilepsy aetiology and severity. These differences in placebo response undermine the validity of indirect comparisons of adjunctive AED trials.
Attempts have been made to overcome cross-trial heterogeneity in baseline patient characteristics by conducting matching-adjusted indirect comparisons (MAIC) [23,24,25]. MAIC methods use individual patient data to adjust for observed baseline characteristics, applying a propensity score-based model to weight patients so the populations are comparable between trials. In this way, trial outcomes can be compared across balanced populations. Differences in outcomes in weighted placebo arms may indicate residual confounding not accounted for by propensity score weighting.
At present, it is unclear if indirect comparison methods, and MAIC methods in particular, are suitable for accurately generating comparative efficacy evidence of the adjunctive use of brivaracetam versus levetiracetam. Thus, the objective of this study was to determine whether it is possible to successfully adjust for differences in baseline characteristics to mitigate placebo arm response differences in levetiracetam (recruited during 1993–1998) and brivaracetam (recruited during 2007–2014) trials using a MAIC study design.
2 Methods
2.1 Trial Selection
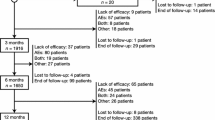
This analysis included phase III clinical trials of oral levetiracetam or brivaracetam as adjunctive therapy enrolling patients with focal epilepsy and focal seizure frequency of four or more (averaged over 28 days) uncontrolled on one to three concomitant AEDs. Patient data were retrieved from previously published clinical studies; thus, no institutional review was required.
2.1.1 Study Identification
To identify non-UCB Pharma-conducted adjunctive levetiracetam trials, a previous systematic literature review was used [databases searched included Ovid MEDLINE (1946–1 August 2012), Ovid EMBASE (1974–31 July 2012), Ovid PsycINFO (1806–week of 5 July 2012), OvidEconLit (1961–June 2012), Health Economic Evaluation Database (HEED; to 1 August 2012), Cochrane Controlled Trials Register (to 1 August 2012)] [26]. To cover the most recent years, a new systematic literature review of double-blind, placebo-controlled (non-UCB Pharma) trials of oral adjunctive therapy with levetiracetam for focal seizures was performed in October 2013 [PRISMA; Electronic Supplementary Material (ESM) Online Resource 1]. The review assessed studies published in 2012/2013 from MEDLINE, EMBASE, PsycInfo, Cochrane Library and ClinicalTrials.gov. Non-UCB Pharma levetiracetam trial investigators were contacted to request individual patient data, without response. Only trials conducted by the manufacturer were available for brivaracetam at the time of this analysis, thus all data were supplied without the need for a systematic review of published literature. As such, the manufacturer (UCB Pharma) supplied all data for levetiracetam and brivaracetam.
2.1.2 Inclusion and Exclusion Criteria
Included trials were required to have patient-level data available, a treatment duration of at least 12 weeks, and include approved doses for the treatments (levetiracetam 1000–3000 mg/day; brivaracetam 50–200 mg/day) with a titration period permitted. In cases where otherwise eligible studies included arms with unapproved doses, those arms were excluded. Trials studying non-oral or extended-release formulations or monotherapy levetiracetam/brivaracetam, and dosages outside of the approved dosage range (or flexible dosing for brivaracetam), were excluded. In addition, trials of non-human subjects, healthy patients, patients with other forms of epilepsy (e.g. generalised), patients aged <16 years or treatment-naïve patients were excluded. Brivaracetam is not currently approved in Asia; thus, studies conducted exclusively in Asia were excluded.
2.1.3 Outcomes
Included trials were required to report the following efficacy outcomes at 12 weeks from baseline: median percentage reduction in focal seizure frequency from baseline to the treatment period; and percentage achieving a ≥50% reduction in focal seizure frequency (responder rate) during the treatment period.
2.2 Patient Selection
The potential sample consisted of adult patients enrolled in one of the six randomised, double-blind, phase III efficacy and safety studies that met the selection criteria (brivaracetam trials: N01252/NCT00490035 [27], N01253/NCT00464269 [28] and N01358/NCT01261325 [29]; levetiracetam trials: N051 [30], N132 [31] and N138 [32]). Consistent enrolment criteria were applied across populations for the current analysis (Table 1).
This study included patients in the intent-to-treat (ITT) populations, i.e. those who were randomised and received at least one dose of the study medication, as reported in the original trials. Common criteria for patient inclusion were as follows: (1) aged 16–65 years; (2) well-characterised focal epilepsy, with or without secondary generalization; (3) uncontrolled focal seizures while on a stable dose of one to three concomitant AEDs; (4) randomised to treatment with placebo or approved brivaracetam/levetiracetam dosages; and (5) four or more focal seizures averaged over 28 days during the baseline period.
Common criteria for patient exclusion were as follows: (1) a diagnosis of primary generalised epilepsy; (2) seizure clusters prior to study entry or during the baseline period; or (3) taking medications that influenced the central nervous system. In addition, patients with status epilepticus in the 12 months prior to randomisation (or at any time in trial N051 [30]) were excluded, and patients receiving concomitant levetiracetam were excluded from brivaracetam trials N01252 [27] and N01253 [28].
2.3 Study Design and Outcomes
The populations for analysis were pooled across trials by drug. Patient characteristics were assessed during the baseline period. For levetiracetam trials N132 [31] and N138 [32], the baseline period was defined as the 8 weeks prior to randomisation, which created an 8-week baseline period consistent with the other four trials. AED history was not analysed, as it was assessed over different time horizons across the trials. The treatment period was defined as the 12 weeks after randomisation. Efficacy outcomes in the placebo-treated patients were assessed from randomisation to Week 12 of the treatment period (which, for levetiracetam trials, included a titration period). Levetiracetam trial N132 [31] did not have a Week 12 visit; therefore, the average of the assessments for Weeks 10–14 was used in place of these data. Efficacy outcomes included median percentage reduction from baseline in focal seizure frequency (averaged over 28 days), and ≥50% responder rate (defined as the percentage of patients who achieved ≥50% reduction in focal seizure frequency from baseline to the first 12 weeks of the treatment period).
2.4 Statistical Analyses
Baseline characteristics were compared using chi-squared tests for binary variables and t tests for continuous variables. Propensity score weighting was used to adjust for potentially confounding baseline characteristics between the brivaracetam and levetiracetam trial populations not accounted for by use of consistent inclusion/exclusion criteria and assessment of outcomes over the same time period. The propensity for enrolment in the brivaracetam versus levetiracetam trials was estimated using a multivariate logistic regression model, where baseline characteristics were independent variables. Several propensity score models were evaluated for model fit (e.g. defining variables as continuous versus categorical, such as the current number of AEDs, and including anxiety and depression in the models) and the model with the best fit was selected. Model fit (i.e. calibration) was assessed using the Hosmer–Lemeshow goodness-of-fit test [33]; overlap in the propensity score distributions was also assessed to avoid extrapolation. Patients were weighted by their inverse propensity score. Following weighting, brivaracetam/levetiracetam population balance was assessed by comparing baseline characteristics between the weighted populations. The Hosmer–Lemeshow goodness-of-fit test, assessment of overlap in brivaracetam and levetiracetam propensity scores, and a comparison of differences of post-weighting baseline characteristics were used to assess weighting success.
Means with standard deviations (SDs), and medians and percentages with 95% confidence intervals (CIs) were reported. Medians were compared using quantile regressions; significance was denoted at p ≤ 0.05. Statistical analyses were conducted in STATA® 13 (StataCorp LP, College Station, TX, USA) software.
3 Results
The analysis included 1832 patients, composed of 707 and 473 active drug and 399 and 253 placebo in adjunctive brivaracetam and levetiracetam trials, respectively (Table 1) [27,28,29,32]. Among the levetiracetam trials, 35.1% of patients were located in North America, 46.4% in Western Europe and 18.5% in Eastern Europe (ESM Online Resource 2). Among the brivaracetam trials, 29.1% of patients were in North America, 27.4% in Western Europe, 23.7% in Eastern Europe, 13.2% in Asia and 6.4% in another region (‘Other’).
Prior to weighting, brivaracetam and levetiracetam trial patients had several significantly different baseline characteristics, including demographics (age, race, region and height), seizure type and frequency [focal seizures secondarily generalised (Type IC seizures) and median number of focal seizures/28 days], epilepsy history (the age at onset of first seizures and vagal nerve stimulation), number of concomitant AEDs (one/two AEDs), co-morbid anxiety, co-morbid depression and aetiology (Tables 2, 3). Specifically, a smaller proportion of brivaracetam trial patients were Caucasian than levetiracetam trial patients (73 vs. 95%, respectively; p < 0.01), brivaracetam trial patients had more concomitant AEDs on average [1.80 (SD 0.49) vs. 1.56 (0.56); p < 0.01], and more brivaracetam patients received vagal nerve stimulation (6 vs. 0%; p < 0.01; Table 2). Fewer levetiracetam than brivaracetam trial patients had co-morbid anxiety (2 vs. 8%, respectively) or co-morbid depression (7 vs. 14%; both p < 0.01) or evidence of a structural lesion (31 vs. 36%; p < 0.05) at baseline (Table 2). A lower proportion of brivaracetam than levetiracetam trial patients had Type IB seizures at baseline (82 vs. 91%, respectively; p < 0.01), but a higher proportion had Type IA (38 vs. 33%) and IC (30 vs. 26%; both p < 0.05; Table 3). In addition, mean baseline Type IC seizure frequency/28 days were greater for brivaracetam than for levetiracetam trial patients [1.46 (SD 3.95) vs. 1.01 (35.55), respectively], as was median baseline focal seizure frequency/28 days [9.59 (range 5.82–22.30) vs. 9.64 (6.43–19.31); both p < 0.01; Table 3]. Before weighting, median percentage reduction from baseline in focal seizure frequency was 21.7% (95% CI 14.8–28.7) for brivaracetam and 3.9% (–2.7 to 10.5) for levetiracetam trial placebo arm patients (Fig. 1), and ≥50% responder rates were 22.0% (16.7–28.4) and 9.5% (6.4–13.9), respectively (both p < 0.001; Fig. 2).
The baseline characteristics included in the propensity score model are indicated using a superscripted letter ‘a’ in Tables 2 and 3. These included demographics (age, weight, height, race, sex, region), epilepsy history [duration of epilepsy (years), age at seizure onset], number of concomitant AEDs, presence of a potential structural lesion, type of baseline seizures and median baseline focal seizure frequency/28 days. Co-morbid depression and co-morbid anxiety were not included in the model to improve the fit. The model demonstrated good fit with no evidence of poor calibration, based on the non-significant result from the Hosmer–Lemeshow goodness-of-fit test (p = 0.20).
After weighting, patient demographics and baseline characteristics and baseline seizure profiles (Table 3) appeared similar for pooled brivaracetam and levetiracetam trial populations, with the exception of vagal nerve stimulation (6 vs. 0%, respectively; p < 0.01), co-morbid depression and co-morbid anxiety. However, the median percentage reduction in focal seizure frequency in placebo-treated patients from brivaracetam and levetiracetam trials remained different after weighting [15% (95% CI 10.2–19.8) vs. 6.0% (1.7–10.4); p < 0.05], with placebo response 2.5 times higher among patients in brivaracetam versus levetiracetam trials compared with 5.6 times higher before weighting (Fig. 1). After weighting, the difference in ≥50% responder rates in placebo-treated patients from brivaracetam and levetiracetam trials was somewhat reduced [16.3% (95% CI 11.1–23.4) vs. 12.3% (5.7–24.4); p = 0.56], with placebo response 1.3 times higher in brivaracetam versus levetiracetam trials, compared with 2.3 times higher before weighting (Fig. 2).
4 Discussion
This study tested the possibility of using the MAIC method to compare the efficacy of adjunctive brivaracetam and levetiracetam across clinical trials. We compared placebo-treated brivaracetam and levetiracetam trial arms following propensity score weighting used to generate comparable patient groups at baseline. The results of the MAIC showed that weighting mitigated only approximately half the difference in placebo response between brivaracetam and levetiracetam trial patients. After adjustment, median percentage reduction in seizure frequency and ≥50% responder rates in the placebo arm for brivaracetam remained 2.5 and 1.3 times higher, respectively, than for levetiracetam. Thus, the MAIC methodology did not successfully balance placebo response across these adjunctive trials, bringing into question the validity of using indirect comparisons to assess AED efficacy using trials from different eras, especially when no attempt is made to adjust for heterogeneity.
These conclusions are consistent with those of prior indirect comparisons of adjunctive AED trial data applying similar techniques. A study by Rheims et al. [20] evaluated 63 randomised clinical trials (RCTs) of 20 adjunctive AEDs and reported that both AED and placebo responder rates have increased over the years, doubling between 1989 and 2009. The trend remained significant only for placebo responders after linear regression, and was associated with a non-significant trend of lowered active arm response. Rheims et al. [20] hypothesised that differing or changing patient characteristics over time may contribute to this parallel increase in response. Similar trends have been noted for RCTs of bipolar mania [34], major depression [35, 36] and schizophrenia [37]. Conversely, a 2010 random effects meta-analysis of 27 adjunctive AED trials reported a non-significant response rate increase over time (p = 0.064), and found no significant correlation of placebo response rate with active arm response rate or baseline median seizure frequency [38].
While increasing response rates to placebo have been observed in recent adjunctive AED RCTs in adults with epilepsy, compared to prior eras [38], there exists a wide variation in placebo responder rates even between trials close in time. RCTs of AEDs for refractory focal seizures published in 2008–2010 had rates that varied from 9.9 to 39% [21, 22]. Additionally, greater treatment arm responder rates have been noted in trials with longer evaluation periods [20].
Many potential explanations exist for heterogeneity across adjunctive AED trials, including chance, more reliable AED intake in later years [39], regression to the mean or the natural history of epilepsy [40], observer bias, length of the trial and ≥12-week versus <12-week follow-up periods (longer are more likely to confirm extended efficacy), or the geographic location of trial [41,42,43,43]. In addition to general patient bias and/or desire to remain in the trial, patients in poorer countries may have further incentive to stay in the trial or report improvements to receive care [44]. Similarly, receiving a higher level of care or a reduction in stress during the trial may influence patients’ seizure susceptibility [45]. Trial populations have also changed. Patients in more recent trials have exhibited higher refractoriness and severity of epilepsy at baseline, and poorer AED responses are associated with high seizure frequency and a longer duration of epilepsy [46]. Patients have more concomitant and lifetime AEDs in recent years, and the heterogeneity of concomitant AEDs in older versus more recent trials has been noted [47, 48]. The availability of AEDs has expanded over time, providing more treatment choice. Thus, newer-generation AEDs have less chance to show efficacy than older AEDs as they may be given to drug-resistant patients who have already failed to respond or lost response to prior AED therapies [49].
Additionally, the characteristics of trials have evolved. Seizure diaries, a type of patient-reported outcome, have transitioned from paper to electronic format [50]. Studies performed in the 1990s were often conducted at fewer but more experienced, resource-intensive centres [51]. A lower level of recruitment in more recent trials, perhaps due to more approved therapies and lowered likelihood of trying a new therapy, may also affect responder rates. Rates of response may also depend on the ways they are calculated and over what length of time (e.g. seizure frequency compared to baseline could be assessed during the maintenance or entire treatment period). For patients in placebo, but not active treatment, arms, responder rates tend to be higher during the maintenance versus the entire treatment period [20]. Variation exists on whether ITT basis versus last observation carried forward [52] is used, although ITT is preferred by the European Medicines Agency for epilepsy [53]. Furthermore, as the definitions of co-morbidities or epilepsy aetiology evolve, matching on these characteristics (in indirect comparisons) becomes more difficult. Epilepsy is a heterogeneous disorder, which is reflected in treatment response in clinical trials [54]. The classification of epilepsy aetiology (and syndromes and seizure types) is continually evolving under the guidelines of the International League Against Epilepsy [55].
Other than being separated by 10 years, several additional important differences existed among trials in the current analysis. First, historical AED use was assessed over different time horizons in the brivaracetam and levetiracetam trials and thus was excluded as a variable in the propensity score model. Second, there were differing titration periods: the levetiracetam trials included a titration period, whereas the brivaracetam trials did not; this is consistent with their respective labelling. Third, the permitted concomitant use of AEDs varied across trials, which may impact the efficacy results. Specifically, levetiracetam trials N132 [31] and N051 [30], and all three brivaracetam trials, permitted patients to use one to two concomitant AEDs, but levetiracetam trial N138 [32] permitted patients to use only one concomitant AED. On average, levetiracetam patients were using fewer concomitant AEDs than brivaracetam patients. The most commonly used concomitant AEDs differed across the trials; common AEDs in the brivaracetam trials included topiramate, oxcarbazepine and zonisamide. Conversely, common AEDs in the levetiracetam trials included gabapentin, vigabatrin and primidone. Fourth, the patients in the brivaracetam trials had alternate epilepsy treatments available, such as vagal nerve stimulation and epilepsy surgery, which were not as frequently used during the 1990s (levetiracetam trials period). This is reflected in the current finding that 6% of patients in brivaracetam trials versus 0% in levetiracetam trials had received vagal nerve stimulation. Sixth, the studies relied on self-reporting of seizures, and patient diaries have also changed over time [50]. Although the present analysis adjusted for many of these factors, residual confounding was still a significant issue, impacting the ability to effectively compare AED efficacy trials.
In addition to the above differences, the trials were conducted among different regions: the levetiracetam trials were primarily conducted in the USA and Western Europe whereas the brivaracetam trials had a more global distribution. Given the poor overlap, the categories used for region in the propensity score model were ‘North America’ and ‘Other’. In order to improve regional overlap between the brivaracetam and levetiracetam study pools and assure relevance of the results to clinical practice, studies conducted exclusively in Asia, where brivaracetam is currently not registered, were excluded. This led to the exclusion of three levetiracetam studies for which patient-level data were available: Tsai et al. [56], recruited in 2000–2002, where placebo showed a median percentage reduction in seizure frequency of 15.6% (n = 47); Wu et al. [57], recruited 2004–2005, where placebo showed a median percentage reduction in seizure frequency of 13.7% (n = 100); and Inoue et al. [58], recruited 2005–2007, where placebo showed a median percent reduction in seizure frequency of 12.5% (n = 70). Regional differences in placebo response have been reported in adjunctive trials of other AEDs, such as in a phase III study of perampanel by French et al. [43]. In that study, patients from Latin America were observed to have a significant treatment-by-region interaction and necessitated exclusion from the final analysis of efficacy [43].
Indirect comparisons are commonly used to compare drug efficacy, and the method used in the current analysis (MAIC) is a commonly used approach [23,24,25]. However, the myriad of both between- and within-trial heterogeneity issues outlined here may lead to a predictability gap between AED efficacy in adjunctive trials and AED effectiveness in clinical practice [59]. Therefore, questions have been raised about the suitability and generalisability of indirect comparisons of adjunctive AED clinical trials to support comparative claims in clinical, guideline and reimbursement decision-making, undue reliance on which may "inappropriately limit" patients' choices based on the overall average effect on the measured outcomes [54]. As an example, vigabatrin was reported to be a comparatively safe and efficacious drug in a network meta-analysis [19], a finding that would be “[unacceptable] as such by experienced epilepsy clinicians” [49].
The current results indicate a failure of this type of indirect comparison to properly mitigate placebo response differences in brivaracetam and levetiracetam trials, suggesting that indirect comparison methodology may be inappropriate because the populations are not comparable. Thus, indirect comparisons of the efficacy of adjunctive brivaracetam and levetiracetam should be interpreted with caution. Recent attempts to compare efficacy and tolerability of brivaracetam and levetiracetam that have found no significant differences in efficacy [60] may be limited by reliance on placebo anchors and use of clinical trial as opposed to patient-level data. Given the findings of the current study, indirect comparisons based on placebo anchors will not accurately reflect differences in treatment effects between brivaracetam and levetiracetam, and studies based on published trials (as opposed to patient data) may introduce confusion originating from differences across trials such as differing inclusion and exclusion criteria, baseline characteristics and assessed outcomes.
5 Limitations
The findings of this study should be interpreted in light of several limitations. First, propensity score weighting can only adjust for observed patient baseline characteristics; there is still potential for residual confounding due to unobserved differences, which is a main finding of this work. Only head-to-head randomised trials can avoid this limitation. Second, the levetiracetam trials included a titration period, consistent with levetiracetam labelling, whereas the brivaracetam trials did not. To mimic real-world clinical decision-making during the first 12 weeks of treatment, this analysis included the titration period in the evaluation period for levetiracetam outcomes. Fourth, the average of the assessments for Weeks 10–14 was used for levetiracetam trial N132 [31], which may not accurately reflect the first 12 weeks of treatment. However, differences are not expected to be substantial. Finally, a small number of trials (six) met the study selection criteria and were included in the analysis; thus, the conclusions of this study apply to this subset of included brivaracetam and levetiracetam trials. Extrapolation to other trial populations should be made with caution.
6 Conclusion
This study showed that placebo arm differences in adjunctive brivaracetam and levetiracetam epilepsy drug trials indicate the presence of unobserved confounding factors associated with placebo response. These differences suggest that placebo arms from different epilepsy trials, particularly those conducted in different eras, should not be assumed to represent ‘common comparator arms’, as required for network meta-analyses or other anchor-based indirect comparisons. Therefore, it remains problematic to conduct indirect comparisons—whether adjusted, unadjusted or anchor-based—between brivaracetam and levetiracetam using available trial data. These findings may also apply to clinical trials of other AEDs. Approaches should be developed to better account for the heterogeneous nature of epilepsy and the need for individualised patient care in order to have greater value in healthcare decision-making, particularly when comparing older and newer treatments.
References
Institute of Medicine of the National Academies. Epilepsy across the spectrum: promoting health and understanding. Washington, DC: The National Academies Press; 2012.
World Health Organization. Fact sheet: epilepsy. 2017. http://www.who.int/mediacentre/factsheets/fs999/en/. Accessed 21 Jun 2017.
Epilepsy Fast Facts. Center for Disease Control and Prevention. Available from: http://www.cdc.gov/epilepsy/basics/fast-facts.htm. Accessed 28 Jun 2016.
World Health Organization. Epilepsy in the WHO European region 2008. http://www.who.int/mental_health/neurology/epilepsy/euro_report.pdf. Accessed 21 Jun 2017.
Kwan P, Brodie MJ. Effectiveness of first antiepileptic drug. Epilepsia. 2001;42(10):1255–60.
Kaminski RM, Matagne A, Leclercq K, Gillard M, Michel P, Kenda B, et al. SV2A protein is a broad-spectrum anticonvulsant target: functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology. 2008;54(4):715–20.
Sills GJ. Mechanisms of action of antiepileptic drugs. In: Potschka H, Lerche H, editors. Therapeutic targets and perspectives in the pharmacological treatment of epilepsy. Bremen: UNI-MED; 2013. p. 62–5.
Matagne A, Margineanu DG, Kenda B, Michel P, Klitgaard H. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol. 2008;154(8):1662–71.
Abou-Khalil B. Levetiracetam in the treatment of epilepsy. Neuropsychiatr Dis Treat. 2008;4(3):507–23.
Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900.
Tudur Smith C, Marson AG, Chadwick DW, Williamson PR. Multiple treatment comparisons in epilepsy monotherapy trials. Trials. 2007;8:34.
Beyenburg S, Stavem K, Schmidt D. Placebo-corrected efficacy of modern antiepileptic drugs for refractory epilepsy: systematic review and meta-analysis. Epilepsia. 2010;51(1):7–26.
Beyenburg S, Stavem K, Schmidt D. Placebo-corrected efficacy of modern nonenzyme-inducing AEDs for refractory focal epilepsy: systematic review and meta-analysis. Epilepsia. 2012;53(3):512–20.
Hemery C, Ryvlin P, Rheims S. Prevention of generalized tonic-clonic seizures in refractory focal epilepsy: a meta-analysis. Epilepsia. 2014;55(11):1789–99.
Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91.
Brigo F, Trinka E, Bragazzi NL, Nardone R, Milan A, Grillo E. A common reference-based indirect comparison meta-analysis of eslicarbazepine versus lacosamide as add on treatments for focal epilepsy. Epilepsy Res. 2016;127:12–8.
Otoul C, Arrigo C, Rijckevorsel KV, French JA. Meta-analysis and indirect comparisons of levetiracetam with other second-generation antiepileptic drugs in partial epilepsy. Clin Neuropharmacol. 2005;28(2):72–8.
Costa J, Fareleira F, Ascencao R, Borges M, Sampaio C, Vaz-Carneiro A. Clinical comparability of the new antiepileptic drugs in refractory partial epilepsy: a systematic review and meta-analysis. Epilepsia. 2011;52(7):1280–91.
Bodalia PN, Grosso AM, Sofat R, Macallister RJ, Smeeth L, Dhillon S, et al. Comparative efficacy and tolerability of anti-epileptic drugs for refractory focal epilepsy: systematic review and network meta-analysis reveals the need for long term comparator trials. Br J Clin Pharmacol. 2013;76(5):649–67.
Rheims S, Perucca E, Cucherat M, Ryvlin P. Factors determining response to antiepileptic drugs in randomized controlled trials. A systematic review and meta-analysis. Epilepsia. 2011;52(2):219–33.
Xiao Z, Li JM, Wang XF, Xiao F, Xi ZQ, Lv Y, et al. Efficacy and safety of levetiracetam (3000 mg/Day) as an adjunctive therapy in Chinese patients with refractory partial seizures. Eur Neurol. 2009;61(4):233–9.
Zaccara G, Giovannelli F, Schmidt D. Placebo and nocebo responses in drug trials of epilepsy. Epilepsy Behav. 2015;43:128–34.
Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–7.
Kirson NY, Rao S, Birnbaum HG, Kantor E, Wei RS, Cifaldi M. Matching-adjusted indirect comparison of adalimumab vs etanercept and infliximab for the treatment of psoriatic arthritis. J Med Econ. 2013;16(4):479–89.
Signorovitch JE, Wu EQ, Swallow E, Kantor E, Fan L, Gruenberger JB. Comparative efficacy of vildagliptin and sitagliptin in Japanese patients with type 2 diabetes mellitus: a matching-adjusted indirect comparison of randomized trials. Clin Drug Investig. 2011;31(9):665–74.
Charokopou M, Harvey R, Srivastava K, Townsend R, Borghs S. Applying the accepted techniques of indirect treatment comparison to meet payer needs for brivaracetam versus adjunctive antiepileptic medications for the treatment of focal seizures in adult patients. Do they reflect the need for individualised patient care in heterogeneous populations in epilepsy? [abstract presented as poster]. ISPOR-EU; 29 Oct–2 Nov 2016; Vienna.
Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55(1):47–56.
Biton V, Berkovic SF, Abou-Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55(1):57–66.
Klein P, Schiemann J, Sperling MR, Whitesides J, Liang W, Stalvey T, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56(12):1890–8.
Shorvon SD, Lowenthal A, Janz D, Bielen E, Loiseau P. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. European Levetiracetam Study Group. Epilepsia. 2000;41(9):1179–86.
Cereghino JJ, Biton V, Abou-Khalil B, Dreifuss F, Gauer LJ, Leppik I. Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology. 2000;55(2):236–42.
Ben-Menachem E, Falter U. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia. 2000;41(10):1276–83.
Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed. Hoboken: Wiley; 2013.
Sysko R, Walsh BT. A systematic review of placebo response in studies of bipolar mania. J Clin Psychiatry. 2007;68(8):1213–7.
Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–7.
Stolk P, Ten Berg MJ, Hemels ME, Einarson TR. Meta-analysis of placebo rates in major depressive disorder trials. Ann Pharmacother. 2003;37(12):1891–9.
Chen YF, Wang SJ, Khin NA, Hung HM, Laughren TP. Trial design issues and treatment effect modeling in multi-regional schizophrenia trials. Pharm Stat. 2010;9(3):217–29.
Guekht AB, Korczyn AD, Bondareva IB, Gusev EI. Placebo responses in randomized trials of antiepileptic drugs. Epilepsy Behav. 2010;17(1):64–9.
Cramer J, Vachon L, Desforges C, Sussman NM. Dose frequency and dose interval compliance with multiple antiepileptic medications during a controlled clinical trial. Epilepsia. 1995;36(11):1111–7.
Goldenholz DM, Moss R, Scott J, Auh S, Theodore WH. Confusing placebo effect with natural history in epilepsy: a big data approach. Ann Neurol. 2015;78(3):329–36.
Baulac M, Leon T, O’Brien TJ, Whalen E, Barrett J. A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partial-onset seizures. Epilepsy Res. 2010;91(1):10–9.
Kramer LD, Satlin A, Krauss GL, French J, Perucca E, Ben-Menachem E, et al. Perampanel for adjunctive treatment of partial-onset seizures: a pooled dose-response analysis of phase III studies. Epilepsia. 2014;55(3):423–31.
French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79(6):589–96.
Friedman D, French JA. Clinical trials for therapeutic assessment of antiepileptic drugs in the 21st century: obstacles and solutions. Lancet Neurol. 2012;11(9):827–34.
Furmark T, Appel L, Henningsson S, Ahs F, Faria V, Linnman C, et al. A link between serotonin-related gene polymorphisms, amygdala activity, and placebo-induced relief from social anxiety. J Neurosci. 2008;28(49):13066–74.
Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs: effect of past treatment history. Neurology. 2008;70(1):54–65.
Khan N, Shah D, Tongbram V, Verdian L, Hawkins N. The efficacy and tolerability of perampanel and other recently approved anti-epileptic drugs for the treatment of refractory partial onset seizure: a systematic review and Bayesian network meta-analysis. Curr Med Res Opin. 2013;29(8):1001–13.
Zaccara G, Giovannelli F, Maratea D, Fadda V, Verrotti A. Neurological adverse events of new generation sodium blocker antiepileptic drugs. Meta-analysis of randomized, double-blinded studies with eslicarbazepine acetate, lacosamide and oxcarbazepine. Seizure. 2013;22(7):528–36.
Zaccara G, Giovannelli F, Bell GS, Sander JW. Network meta-analyses of antiepileptic drug efficacy and tolerability in drug-resistant focal epilepsies: a clinical perspective. Eur J Clin Pharmacol. 2014;70(6):647–54.
Fisher RS, Blum DE, DiVentura B, Vannest J, Hixson JD, Moss R, et al. Seizure diaries for clinical research and practice: limitations and future prospects. Epilepsy Behav. 2012;24(3):304–10.
Ravina B. Clinical trials in neurology : design, conduct, analysis. Cambridge: Cambridge University Press; 2012.
Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia. 2007;48(7):1303–7.
European Medicines Agency. Guideline on clinical investivation of medicinal products in the treatment of epileptic disorders. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070043.pdf. Accessed12 Jul 2016.
French JA, England JD. Invited Article: Comparative effectiveness research, evidence-based medicine, and the AAN. Neurology. 2010;75:562–567.
International League Against Epilepsy. Definition and classification. 2014. http://www.ilae.org/visitors/centre/Definition_Class.cfm. Accessed on: July 11, 2016.
Tsai JJ, Yen DJ, Hsih MS, Chen SS, Hiersemenzel R, Edrich P, et al. Efficacy and safety of levetiracetam (up to 2000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double-blind, placebo-controlled study. Epilepsia. 2006;47(1):72–81.
Wu XY, Hong Z, Wu X, Wu LW, Wang XF, Zhou D, et al. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in Chinese patients with refractory partial-onset seizures. Epilepsia. 2009;50(3):398–405.
Inoue Y, Yagi K, Ikeda A, Sasagawa M, Ishida S, Suzuki A, et al. Efficacy and tolerability of levetiracetam as adjunctive therapy in Japanese patients with uncontrolled partial-onset seizures. Psychiatry Clin Neurosci. 2015;69(10):640–8.
Walker MC, Sander JW. Difficulties in extrapolating from clinical trial data to clinical practice: the case of antiepileptic drugs. Neurology. 1997;49(2):333–7.
Zhang L, Li S, Li H, Zou X. Levetiracetam vs. brivaracetam for adults with refractory focal seizures: a meta-analysis and indirect comparison. Seizure. 2016;39:28–33.
Acknowledgements
Medical writing assistance was provided by Shelley Batts, PhD, an employee of Analysis Group, Inc. In collaboration with all authors, Dr. Batts assisted with literature review, writing the first draft, revising the manuscript and addressing reviewers’ comments.
Author information
Authors and Affiliations
Contributions
The study sponsor was involved in all stages of the study research and manuscript preparation, but all authors participated in the design of the study and contributed to the manuscript development. Data were collected by Analysis Group and analysed and interpreted in collaboration with all other authors. Manuscript drafts were prepared by the authors with editorial assistance from a professional medical writer, Shelley Batts, PhD, ultimately paid by the sponsor, UCB Pharma. All the authors vouch for the accuracy and completeness of the data reported and the adherence of the study to the protocol, and all the authors made the decision to submit the manuscript for publication.
Corresponding author
Ethics declarations
Funding
Financial support for this study was provided entirely by a contract with UCB Pharma. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing and publishing the report. The publisher’s open access fee was paid by the sponsor, UCB Pharma. UCB Pharma Limited is a company registered in England and Wales with registered number 00209905, with a registered office at 208 Bath Road, Slough, Berkshire SL1 3WE.
Conflict of interest
Elyse Swallow, Anna Fang and James Signorovitch are employees of Analysis Group, Inc., which received funding for this research from UCB Pharma. Jonathan Plumb and Simon Borghs are employees of UCB Pharma and own stock/stock options.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Prior presentation
This research has been presented in poster format at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 21st Annual Meeting on 21–25 May 2016 in Washington, DC, USA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Swallow, E., Fang, A., Signorovitch, J. et al. Can Matching-Adjusted Indirect Comparison Methods Mitigate Placebo Response Differences Among Patient Populations in Adjunctive Trials of Brivaracetam and Levetiracetam?. CNS Drugs 31, 899–910 (2017). https://doi.org/10.1007/s40263-017-0462-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-017-0462-8