Abstract
Background
This analysis aimed to characterize the pharmacokinetics (PK) of the inhaled corticosteroid (ICS) fluticasone furoate (FF), the long-acting muscarinic antagonist umeclidinium (UMEC), and the long-acting β2-agonist (LABA) vilanterol (VI), administered as dual (FF/VI) or triple (FF/UMEC/VI) single-inhaler therapy to patients with asthma, and to identify covariates that may influence the PK of each analyte.
Methods
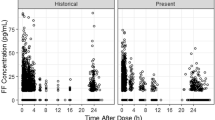
Blood samples were obtained from the phase IIIA CAPTAIN study (ClinicalTrials.gov: NCT02924688), which evaluated the efficacy and safety of once-daily FF/UMEC/VI versus FF/VI in patients with uncontrolled asthma taking ICS/LABA. Samples were collected at trough (defined as ≥ 20 h after the last dose) from all subjects randomized to the six treatment groups (FF/UMEC/VI 100/31.25/25 μg, 100/62.5/25 μg, 200/31.25/25 μg, 200/62.5/25 μg; FF/VI 100/25 μg, 200/25 μg) at week 24 or the early withdrawal visit. In a subset of patients, PK samples were obtained predose at week 12, and at 5–30 min, 45–90 min, and 2–3 h postdose. For each analyte, a population PK model was developed using non-linear mixed-effects modeling. The maximum likelihood method was utilized to incorporate data below the quantifiable limit (BQL). Final models were used to derive the area under the plasma concentration-time curve and maximum observed concentration at steady-state for each analyte.
Results
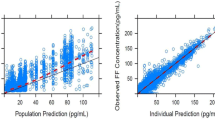
We obtained 4018, 2695, and 4032 samples from 1891, 1258, and 1891 patients, for FF, UMEC, and VI, respectively; 48%, 49%, and 50% of samples were reported as BQL for each analyte, respectively. The PK were adequately described by a two-compartment model with first-order absorption and elimination for FF, a two-compartment model with intravenous bolus input and first-order elimination for UMEC, and a three-compartment model with zero-order input and first-order elimination for VI. Statistically significant covariates were body weight on apparent inhaled clearance of FF, creatinine clearance on apparent clearance and body weight on apparent inhaled volume of distribution of the central compartment for UMEC, and race (East Asian, Japanese, and South East Asian heritage) on inhaled apparent volume of distribution of the central compartment for VI. However, the overall effects of covariates were marginal and thus do not warrant dose adjustment. Systemic exposures of FF or VI did not differ when administered as a single-inhaler triple (FF/UMEC/VI) or dual combination (FF/VI), and were similar to those reported for patients with chronic obstructive pulmonary disease.
Conclusion
Only marginal covariate effects were observed, and thus no dose adjustments are deemed necessary for FF, UMEC, or VI. There was no difference in FF or VI systemic exposure in patients with asthma when administered as either triple (FF/UMEC/VI) or dual therapy (FF/VI). Together with efficacy findings from the CAPTAIN study, our data support the use of single-inhaler FF/UMEC/VI triple therapy for patients with uncontrolled asthma currently receiving ICS/LABA.

Similar content being viewed by others
References
Bernstein DI, Bateman ED, Woodcock A, Toler WT, Forth R, Jacques L, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52(10):1073–83.
Lee LK, Obi E, Paknis B, Kavati A, Chipps B. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55(2):208–19.
Sulaiman I, Greene G, MacHale E, Seheult J, Mokoka M, D’Arcy S, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J. 2018;51(1):1701126.
Davis J, Trudo F, Siddall J, Small M. Burden of asthma among patients adherent to ICS/LABA: a real-world study. J Asthma. 2019;56(3):332–40.
GINA. Global Initiative for Asthma guidelines 2020. https://ginasthma.org/. Accessed 3 Aug 2020.
GSK. Trelegy Ellipta US prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209482s000lbl.pdf. Accessed 8 Jul 2020.
Lee LA, Bailes Z, Barnes N, Boulet L, Edwards D, Fowler A, et al. Efficacy and safety of once-daily single-inhaler triple therapy (fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma: a double-blind, randomised phaseIIIA trial (CAPTAIN study). Lancet Respir Med. 2021;9(1):69–84.
Allen A, Siederer S, Yang S. Population pharmacokinetics of inhaled fluticasone furoate and vilanterol in adult and adolescent patients with asthma. Int J Clin Pharmacol Ther. 2016;54(4):269–81.
Yang S, Lee L, Pascoe S. Population pharmacokinetics modeling of inhaled umeclidinium for adult patients with asthma. Eur J Drug Metab Pharmacokinet. 2017;42(1):79–88.
Mehta R, Farrell C, Hayes S, Birk R, Okour M, Lipson DA. Population pharmacokinetic analysis of fluticasone furoate/umeclidinium bromide/vilanterol in patients with chronic obstructive pulmonary disease. Clin Pharmacokinet. 2020;59(1):67–79.
GOLD. Global Initiative for Chronic Obstructive Lung Disease GOLD Report. 2020. https://goldcopd.org/gold-reports/. Accessed 3 Aug 2020.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35(4):401–21.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Mehta R, Hardes K, Brealey N, Tombs L, Preece A, Kelleher D. Effect of severe renal impairment on umeclidinium and umeclidinium/vilanterol pharmacokinetics and safety: a single-blind, nonrandomized study. Int J Chron Obstruct Pulmon Dis. 2015;10:15–23.
Korotzer B, Ong S, Hansen JE. Ethnic differences in pulmonary function in healthy nonsmoking Asian–Americans and European–Americans. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1101–8.
Goyal N, Beerahee M, Kalberg C, Church A, Kilbride S, Mehta R. Population pharmacokinetics of inhaled umeclidinium and vilanterol in patients with chronic obstructive pulmonary disease. Clin Pharmacokinet. 2014;53(7):637–48.
Acknowledgements
Editorial support in the form of preparation of the first draft based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, was provided by Rebecca Dawson, Ph.D., of Fishawack Indicia, Ltd, UK, part of Fishawack Health, and was funded by GSK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the GlaxoSmithKline (study number GSK 205715), NCT02924688.
Conflict of Interest
SY, NS, AF, and GP are all employees of GlaxoSmithKline (GSK) and own stocks and shares in the company. LAL was an employee of GSK at the time of this study. GP also owns stocks and shares in Novartis.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice, and applicable country-specific regulatory requirements. The protocol received approval from applicable central or local Institutional Review Boards or independent Ethics Committees.
Consent to Participate
Written informed consent was obtained from all participants before participation.
Consent for Publication
Not applicable.
Availability of Data and Material
Anonymized individual participant data and study documents can be requested for further research from http://www.clinicalstudydatarequest.com.
Code Availability
Not applicable.
Author Contributions
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. SY, LL, and GP were involved in the conception and design of the study and the data analysis and interpretation. AF and NS were involved in the data analysis and interpretation.
Additional information
Laurie A. Lee: Affiliation at the time of this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, S., Lee, L.A., Sule, N. et al. Population Pharmacokinetic Modeling of Fluticasone Furoate, Umeclidinium Bromide, and Vilanterol in Patients with Asthma, Using Data from a Phase IIIA Study (CAPTAIN). Clin Pharmacokinet 60, 887–896 (2021). https://doi.org/10.1007/s40262-021-00988-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-00988-1