Abstract
Background
Allometric scaling is often used to describe the covariate model linking total body weight (WT) to clearance (CL); however, there is no consensus on how to select its value.
Objectives
The aims of this study were to assess the influence of between-subject variability (BSV) and study design on (1) the power to correctly select the exponent from a priori choices, and (2) the power to obtain unbiased exponent estimates.
Methods
The influence of WT distribution range (randomly sampled from the Third National Health and Nutrition Examination Survey, 1988–1994 [NHANES III] database), sample size (N = 10, 20, 50, 100, 200, 500, 1000 subjects), and BSV on CL (low 20%, normal 40%, high 60%) were assessed using stochastic simulation estimation. A priori exponent values used for the simulations were 0.67, 0.75, and 1, respectively.
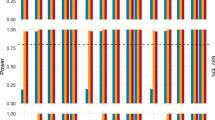
Results
For normal to high BSV drugs, it is almost impossible to correctly select the exponent from an a priori set of exponents, i.e. 1 vs. 0.75, 1 vs. 0.67, or 0.75 vs. 0.67 in regular studies involving < 200 adult participants. On the other hand, such regular study designs are sufficient to appropriately estimate the exponent. However, regular studies with < 100 patients risk potential bias in estimating the exponent.
Conclusion
Those study designs with limited sample size and narrow range of WT (e.g. < 100 adult participants) potentially risk either selection of a false value or yielding a biased estimate of the allometric exponent; however, such bias is only relevant in cases of extrapolating the value of CL outside the studied population, e.g. analysis of a study of adults that is used to extrapolate to children.







Similar content being viewed by others
References
Xu XS, Yuan M, Yang H, Feng Y, Xu J, Pinheiro J. Further evaluation of covariate analysis using empirical Bayes estimates in population pharmacokinetics: the perception of shrinkage and likelihood ratio test. AAPS J. 2017;19(1):264–73.
McLeay SC, Morrish GA, Kirkpatrick CM, Green B. The relationship between drug clearance and body size. Clin Pharmacokinet. 2012;51(5):319–30.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development—part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacomet Syst Pharmacol. 2013;2(4):e38.
Rubner M. Ueber den einfluss der korpergrosse auf stoffund kaftwechsel. Zeitschrift fur Biologie. 1883;19:535–62.
Kleiber M. Body size and metabolism. Hilgardia. 1932;6(11):315–53.
Brody S. Bioenergetics and growth; with special reference to the efficiency complex in domestic animals. New York: Reinhold Publishing Corporation; 1945.
West GB, Woodruff WH, Brown JH. Allometric scaling of metabolic rate from molecules and mitochondria to cells and mammals. Proc Natl Acad Sci. 2002;99(Suppl 1):2473–8.
West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276(5309):122–6.
West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284(5420):1677–9.
Mahmood I, Balian J. Interspecies scaling: predicting clearance of drugs in humans. Three different approaches. Xenobiotica. 1996;26(9):887–95.
Mahmood I, Green MD, Fisher JE. Selection of the first-time dose in humans: comparison of different approaches based on interspecies scaling of clearance. J Clin Pharmacol. 2003;43(7):692–7.
Mahmood I. Interspecies scaling of protein drugs: prediction of clearance from animals to humans. J Pharm Sci. 2004;93(1):177–85.
Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol. 2009;49(12):1382–402.
Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36.
Dodds PS, Rothman DH, Weitz JS. Re-examination of the “3/4-law” of metabolism. J Theor Biol. 2001;209(1):9–27.
White CR, Seymour RS. Mammalian basal metabolic rate is proportional to body mass2/3. Proc Natl Acad Sci. 2003;100(7):4046–9.
He J-H, Zhang J. Fifth dimension of life and the 4/5 allometric scaling law for human brain. Cell Biol Int. 2004;28(11):809–15.
Hu T-M, Hayton WL. Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPS J. 2001;3(4):30–43.
Calvier EA, Krekels EH, Välitalo PA, Rostami-Hodjegan A, Tibboel D, Danhof M, et al. Allometric scaling of clearance in paediatric patients: when does the magic of 0.75 fade? Clin Pharmacokinet. 2017;56(3):273–85.
Eleveld DJ, Proost JH, Absalom AR, Struys MM. Obesity and allometric scaling of pharmacokinetics. Clin Pharmacokinet. 2011;50(11):751–3.
Fisher DM, Shafer SL. Allometry, shallometry! Anesth Analg. 2016;122(5):1234–8.
Anderson B, Holford N. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Ribbing J, Jonsson EN. Power, selection bias and predictive performance of the Population Pharmacokinetic Covariate Model. J Pharmacokinet Pharmacodyn. 2004;31(2):109–34.
La Caze A, Duffull S. Estimating risk from underpowered, but statistically significant, studies: was APPROVe on TARGET? J Clin Pharmacy Ther. 2011;36(6):637–41.
Al-Sallami HS, Cheah SL, Han SY, Liew J, Lim J, Ng MA, et al. Between-subject variability: should high be the new normal? Eur J Clin Pharmacol. 2014;70(11):1403.
Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. https://wwwn.cdc.gov/nchs/nhanes/nhanes3/datafiles.aspx. Accessed 15 July 2017.
CDC Growth Chart. https://www.cdc.gov/growthcharts/html_charts/wtage.htm. Accessed 15 July 2017.
Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):716–23.
Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol. 2006;57(2):191–8.
Peloquin CA, Hadad DJ, Molino LPD, Palaci M, Boom WH, Dietze R, et al. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52(3):852–7.
Petain A, Kattygnarath D, Azard J, Chatelut E, Delbaldo C, Geoerger B, et al. Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin Cancer Res. 2008;14(21):7102–9.
Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size—based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49(9):1012–24.
Friberg LE, Ravva P, Karlsson MO, Liu P. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents and adults. Antimicrob Agents Chemother. 2012;56(6):3032–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jaydeep Sinha, Hesham S. Al-Sallami and Stephen B. Duffull declare no conflicts of interest.
Ethics approval
No ethical approval was required for this simulation-based work.
Funding
This work received no specific funding. Jaydeep Sinha received a doctoral scholarship from the School of Pharmacy, University of Otago, New Zealand, during the course of this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sinha, J., Al-Sallami, H.S. & Duffull, S.B. Choosing the Allometric Exponent in Covariate Model Building. Clin Pharmacokinet 58, 89–100 (2019). https://doi.org/10.1007/s40262-018-0667-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-018-0667-0