Abstract
Background and Objective
Pasireotide (SOM230, Signifor®) is a somatostatin analog approved in a subcutaneous formulation for the treatment of Cushing’s disease. This analysis characterizes the population pharmacokinetics (PopPK) of subcutaneous pasireotide jointly in healthy volunteers (HVs) and Cushing’s disease patients (CDPs), evaluating the effects of age, body size, and population on pasireotide pharmacokinetics.
Methods
The analysis dataset included five phase I studies and one each from phase II and phase III. A three-compartment, linear structural pharmacokinetic model was used. Models were specified a priori that varied in the relationship between HVs and CDPs, and the model with the lowest value of the Bayes Information Criterion (BIC) was selected. It was then used to illustrate various features of pasireotide pharmacokinetics.
Results and Conclusions
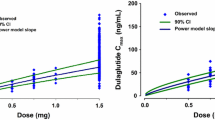
In the final model, the estimated values of apparent clearance (CL/F), central volume of distribution, and deep peripheral volume of distribution of pasireotide in CDP were 59, 43, and 225% those of HVs at the same age and body size. Clearance increased with body size and decreased with age similarly for CDPs and HVs. The estimated CL/F for a typical CDP (40 years old, lean body weight [LBW] 49 kg) was 3.72 L/h, and for a typical HV (29 years old, LBW 61 kg) was 7.96 L/h. The model was judged adequate by visual predictive checks and diagnostic plots separately for HVs and CDPs and can be used for simulations for deriving exposure–response metrics for pharmacokinetic/pharmacodynamic analyses.







Similar content being viewed by others
References
Orth DN. Cushing’s syndrome. N Engl J Med. 1995;332:791–803.
Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BML, Colao A. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016;7:611–29.
Schmid HA. Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74.
Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–16.
McKeage K. Pasireotide: a review of its use in Cushing’s disease. Drugs. 2013;73:563–74.
Signifor label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/200677lbl.pdf. Accessed 29 Dec 2016.
Golor G, Hu K, Ruffin M, Buchelt A, Bouillaud E, Wang Y, et al. A first-in-man study to evaluate the safety, tolerability, and pharmacokinetics of pasireotide (SOM230), a multireceptor-targeted somatostatin analog, in healthy volunteers. Drug Des Devel Ther. 2016;6:71–9.
Beglinger C, Hu K, Wang Y, Bouillaud E, Darstein C, Wang Y, et al. Multiple once-daily subcutaneous doses of pasireotide were well tolerated in healthy male volunteers: a randomized, double-blind, placebo-controlled, cross-over, phase I study. Endocrine. 2012;42:366–74.
Petersenn S, Hu K, Maldonado M, Zhang Y, Lasher J, Bouillaud E, et al. Tolerability and dose proportional pharmacokinetics of pasireotide administered as a single dose or two divided doses in healthy male volunteers: a single-center, open-label, ascending-dose study. Clin Ther. 2012;34:677–88.
Petersenn S, Unger N, Hu K, Weisshaar B, Zhang Y, Bouillaud E, et al. Pasireotide (SOM230), a novel multireceptor-targeted somatostatin analogue, is well tolerated when administered as a continuous 7-day subcutaneous infusion in healthy male volunteers. J Clin Pharmacol. 2012;52:1017–27.
Dietrich H, Hu K, Ruffin M, Song D, Bouillaud E, Wang Y, et al. Safety, tolerability, and pharmacokinetics of a single dose of pasireotide long-acting release in healthy volunteers: a single-center phase I study. Eur J Endocrinol. 2012;166:821–8.
Boscaro M, Ludlam WH, Atkinson B, Glusman JE, Petersenn S, Reincke M, et al. Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab. 2009;94:115–22.
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, et al. Pasireotide B2305 Study Group. A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med. 2012;366:914–24.
Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York: Springer; 2010.
Hallynck TK, Soep HH, Thomis JA, Boelaert J, Daneels R, Dettli L. Should clearance be normalized to body surface or to lean body mass? Br J Clin Pharmacol. 1981;11:523–6.
Zilio M, Barbot M, Ceccato F, Camozzi V, Bilorat F, Casonatot A, et al. Diagnosis and complications of Cushing’s disease: gender-related differences. Clin Endocrin. 2014;80:403–10.
Boxenbaum H, Battle M. Effective half-life in clinical pharmacology. J Clin Pharmacol. 1995;35:763–6.
Teste S, Holford NH, McLachlan AJ. Population pharmacokinetics and pharmacodynamics: an underutilized resource. Drug Inf J. 1998;32:693–710.
Bonate P. Pharmacokinetic-pharmacodynamic modeling and simulation. New York: Springer; 2006.
FDA Office of Clinical Pharmacology. Good review practices: clinical pharmacology review of New Molecular Entity (NME) New Drug Applications (NDAs) and original Biologics License Applications (BLAs). https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/StaffPoliciesandProcedures/ucm073007.pdf. Accessed 11 Jun 2017.
Yu J, Chung S, Zadezensky I, Hu K, Darstein C, Nedelman J, Mehrotra N. Utility of exposure-response analysis in regulatory decision on the selection of starting dose of pasireotide for Cushing disease. J Clin Pharmacol. 2016;56:1035–8.
Acknowledgements
The authors are grateful to the following colleagues who over the years have contributed to the PopPK modelling effort for pasireotide: programming—Vincent Buchheit, Clarisse Chavanne, and Gregory Pinault; and data analysis—Ulrika Waehlby Hamrén and Justin Wilkins.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The studies and analysis reported here were sponsored and performed by Novartis. Jerry Nedelman, Roland Fisch, and Ke Hu were employees of Novartis at the time of this work. The authors agreed to submit the manuscript for publication.
Conflicts of interest
Jerry Nedelman, Roland Fisch, Ke Hu, Ines Paule, and Jocelyn Zhou were or are current employees of Novartis. There is no other conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the individual studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nedelman, J., Fisch, R., Hu, K. et al. Population Pharmacokinetics of Subcutaneous Pasireotide in Healthy Volunteers and Cushing’s Disease Patients. Clin Pharmacokinet 57, 855–866 (2018). https://doi.org/10.1007/s40262-017-0600-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-017-0600-y