Abstract
Background and objective
Peficitinib is an orally administered, once-daily Janus kinase inhibitor in development for the treatment of rheumatoid arthritis. Peficitinib and its major metabolite H2 inhibit the hepatic uptake transporter organic anion transporting polypeptide 1B1 (OATP1B1) in vitro. This article reports a clinical study evaluating the effects of peficitinib on the pharmacokinetics of rosuvastatin, a substrate for the OATP1B1 transporter, and vice versa.
Methods
In an open-label, single-sequence clinical study, 24 healthy adults of East Asian and non-East Asian origin received a single dose of rosuvastatin 10 mg on days 1 and 10. On days 5–13, subjects received a daily dose of 150 mg peficitinib. Serial blood samples for pharmacokinetic assessment of rosuvastatin were collected up to 96 h post-dose on days 1 and 10, and for peficitinib were collected up to 24 h post-dose on days 9 and 10.
Results
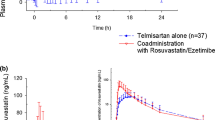
Co-administration of peficitinib with rosuvastatin increased rosuvastatin area under the concentration-time curve (AUC) and maximum plasma concentration (C max) by 18 and 15%, respectively and increased peficitinib AUC and C max by 16 and 28%, respectively. In East Asian (n = 6) vs. non-East Asian subjects (n = 18), peficitinib mean AUC for a dosing interval was 45 and 21% higher, and mean C max was 67 and 34% higher, when administered alone or with rosuvastatin. Peficitinib was well tolerated with few adverse events overall.
Conclusion
In this study, once-daily oral administration of peficitinib had no clinically significant effect on the pharmacokinetics of rosuvastatin, a probe substrate for OATP1B1. Therefore, it is unlikely that peficitinib will have a clinically significant effect on the exposure of other substrates for OATP1B1.
ClinicalTrials.gov number
NCT01959399.


Similar content being viewed by others
References
Takeuchi T, Tanaka Y, Iwasaki M, et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015 K) monotherapy in moderate to severe rheumatoid arthritis patients in Japan: a 12-week, randomized, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75(6):1057–64.
Papp K, Pariser D, Catlin M, et al. A phase 2a randomized, double-blind, placebo-controlled, sequential dose-escalation study to evaluate the efficacy and safety of ASP015K, a novel Janus kinase inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol. 2015;173(3):767–76.
Genovese M, Greenwald M, Codding C, et al. A phase 2b, randomized, double-blind, parallel-group, placebo-controlled, dose-finding, multi-center study to evaluate the safety and efficacy of ASP015K in moderate-to-severe rheumatoid arthritis subjects not on concomitant methotrexate. Arthritis Rheumatol. 2014;66(S10):S1234–5.
Kivitz A, Zubrzycka-Sienkiewicz A, Guttierez-Urena S, et al. A phase 2b, randomized, double-blind, parallel-group, placebo-controlled, dose-finding, multi-center study to evaluate the safety and efficacy of ASP015 K in moderate-to-severe rheumatoid arthritis subjects who have had an inadequate response to methotrexate. Arthritis Rheumatol. 2014;66(S10):S421–2.
Zhu T, Parker B, Wojtkowski T, et al. Drug interactions between ASP015 K (ASP) and rosuvastatin (R) in Asian and non-Asian subjects [abstract no. 1-42-2000353]. Clin Pharmacol Drug Dev. 2014;3(S1):1–59.
Sharma P, Butters CJ, Smith V, et al. Prediction of the in vivo OATP1B1-mediated drug-drug interaction potential of an investigational drug against a range of statins. Eur J Pharm Sci. 2012;47(1):244–55.
Allred AJ, Bowen CJ, Park JW, et al. Eltrombopag increases plasma rosuvastatin exposure in healthy volunteers. Br J Clin Pharmacol. 2011;72(2):321–9.
Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B1 transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81(2):194–204.
Menon RM, Badri PS, Wang T, et al. Drug–drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir, and dasabuvir. J Hepatol. 2015;63(1):20–9.
Prueksaritanont T, Chu X, Evers R, et al. Pitavastatin is a more sensitive and selective organic anion-transporting polypeptide 1B clinical probe than rosuvastatin. Br J Clin Pharmacol. 2014;78(3):587–98.
Davidson M, Ma P, Stein EA, et al. Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. Am J Cardiol. 2002;89(3):268–75.
Davidson MH, Abate N, Ballantyne CM, et al. Ezetimibe/simvastatin compared with atorvastatin or rosuvastatin in lowering to specified levels both LDL-C and each of five other emerging risk factors for coronary heart disease: non-HDL-cholesterol, TC/HDL-C, apolipoprotein B, apo-B/apo-A-I, or C-reactive protein. J Clin Lipidol. 2008;2(6):436–46.
Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152–60.
Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–65.
Simonson SG, Raza A, Martin PD, et al. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther. 2004;76(2):167–77.
Kulmatycki K, Hanna I, Meyers D, et al. Evaluation of a potential transporter-mediated drug interaction between rosuvastatin and pradigastat, a novel DGAT-1 inhibitor. Int J Clin Pharmacol Ther. 2015;53(5):345–55.
Li R, Barton HA, Maurer TS. Toward prospective prediction of pharmacokinetics in OATP1B1 genetic variant populations. CPT Pharmacomet Syst Pharmacol. 2014;3:e151.
Ebner T, Ishiguro N, Taub ME. The use of transporter probe drug cocktails for the assessment of transporter-based drug-drug interactions in a clinical setting: proposal of a four component transporter cocktail. J Pharm Sci. 2015;104(9):3220–8.
Lee E, Ryan S, Birmingham B, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78(4):330–41.
Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–81.
Zhu T, Sawamoto T, Valluri U, et al. Pharmacokinetics, safety, and tolerability of ASP015K, a novel janus kinase inhibitor, in healthy volunteers. Ann Rheum Dis. 2013;72(Suppl 3):A898–9.
MedDRA. Introductory guide MedDRA v14.0. 2011. http://www.meddra.org/sites/default/files/guidance/file/intguide_14_0_english.pdf. [Accessed 17 Jan 2016].
Choi JH, Lee MG, Cho JY, et al. Influence of OATP1B1 genotype on the pharmacokinetics of rosuvastatin in Koreans. Clin Pharmacol Ther. 2008;83(2):251–7.
Smith NF, Figg WD, Sparreboom A. Role of the liver-specific transporters OATP1B1 and OATP1B3 in governing drug elimination. Exp Opin Drug Metab Toxicol. 2005;1(3):429–45.
Acknowledgements
Medical writing support was provided by Matthew Reynolds and Victoria Jones of Choice Healthcare Solutions and funded by Astellas Pharma Inc. We acknowledge all other investigators for their participation in this trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Astellas Pharma Inc.
Conflict of interest
Tong Zhu, Tomasz Wojtkowski, Tetsuya Nishimura, and James Keirns are employees of Astellas Pharma Inc. Barbara Parker, Jay Garg, and Ogert Fisniku were employees of Astellas Pharma Inc. during the study. David Han is an employee of PAREXEL International, contracted by Astellas Pharma Inc. to conduct the clinical assessments throughout this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, T., Parker, B., Wojtkowski, T. et al. Drug Interactions Between Peficitinib, an Orally Administered, Once-Daily Janus Kinase Inhibitor, and Rosuvastatin in Healthy Subjects. Clin Pharmacokinet 56, 747–757 (2017). https://doi.org/10.1007/s40262-016-0474-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0474-4