Abstract
Background and objectives
Vigabatrin is an inhibitor of γ-aminobutyric acid transaminase. The purpose of these analyses was to develop a population pharmacokinetics model to characterize the vigabatrin concentration–time profile for adults and children with refractory complex partial seizures (rCPS) and for children with infantile spasms (IS); to identify covariates that affect its disposition, and to enable predictions of systemic vigabatrin exposure for patients 1–12 months of age.
Methods
Vigabatrin pharmacokinetic data from six randomized controlled clinical trials and one open-label study were analyzed using nonlinear mixed-effects modeling. Data collected from 349 adults with rCPS and 119 pediatric patients with rCPS or IS were used in the analyses.
Results
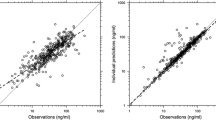
A two-compartment model with first-order elimination and transit-compartment absorption consisting of five transit compartments adequately described the vigabatrin concentration–time data for these adult and pediatric patient populations. An exponential error model was used to estimate inter-individual variability for the transit-rate constant (k tr) (24.2 %), elimination rate constant (k) (14.7 %) and apparent central volume of distribution (V c/F) (18 %). For the study of children with IS, inter-occasion variability was estimated for k tr (58.1 %) and relative bioavailability (F) (26.9 %). Covariate analysis indicated that age, creatinine clearance (CLCR), and body weight were important predictors of vigabatrin pharmacokinetic parameters. Vigabatrin apparent clearance increased with increasing CLCR, consistent with renal excretion (primary pathway of vigabatrin elimination). Rate of vigabatrin absorption was dependent on age. The rate was slower in younger patients, which resulted in a smaller predicted maximum concentration and longer predicted time to maximum concentrations. Vigabatrin V c/F, apparent inter-compartmental clearance between the central and peripheral compartment, and apparent peripheral volume of distribution were increased with greater patient body weights. Sex did not contribute significantly to vigabatrin pharmacokinetic variability.
Conclusion
The model adequately described vigabatrin pharmacokinetic and enabled predictions of systemic exposures in pediatric patients 1–12 months of age.



Similar content being viewed by others
References
Tolman JA, Faulkner MA. Vigabatrin: a comprehensive review of drug properties including clinical updates following recent FDA approval. Expert Opin Pharmacother. 2009;10:3077–89.
Wood JD, Peesker SJ. The anticonvulsant action of GABA-elevating agents: a re-evaluation. J Neurochem. 1975;25:277–82.
Ben-Menachem E. Mechanism of action of vigabatrin: correcting misperceptions. Acta Neurol Scand. 2011;124(Suppl 192):5–15.
Sabril (vigabatrin) prescribing information. Deerfield: Lundbeck, October 2013.
Fraught E. Vigabatrin therapy for refractory complex partial seizures: review of clinical trial experience in the United States. Acta Neurol Scand. 2011;124(Suppl 192):29–35.
Ben-Menachem E, Sander JW. Vigabatrin therapy for refractory complex partial seizures: review of major European trials. Acta Neurol Scand. 2011;124(Suppl 192):16–28.
Hoke JF, Yuh L, Antony KK, et al. Pharmacokinetics of vigabatrin following single and multiple oral doses in normal volunteers. J Clin Pharmacol. 1993;33:458–62.
Frisk-Holmberg M, Kerth P, Meyer P. Effect of food on the absorption of vigabatrin. Br J Clin Pharmacol. 1989;27(Suppl 1):23S–5S.
Durham SL, Hoke JF, Chen TM. Pharmacokinetics and metabolism of vigabatrin following a single oral dose of [14C]vigabatrin in healthy male volunteers. Drug Metab Dispos. 1993;21:480–4.
French JA, Mosier M, Walker S, et al. A double-blind, placebo-controlled study of vigabatrin three g/day in patients with uncontrolled complex partial seizures. Neurology. 1996;46:54–61.
Dean C, Mosier M, Penry K. Dose-response study of vigabatrin as add-on therapy in patients with uncontrolled complex partial seizures. Epilepsia. 1999;40:74–82.
Rey E, Pons G, Richard MO, et al. Pharmacokinetics of the individual enantiomers of vigabatrin (gamma-vinyl GABA) in epileptic children. Br J Clin Pharmacol. 1990;30:253–7.
Kowalski KG, Hutmacher MM. Efficient screening of covariates in population models using Wald’s approximation to the likelihood ratio test. J Pharmacokinet Pharmacodyn. 2001;28:253–76.
Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokin Pharmacodyn. 2001;28:171–92.
Pellock JM, Faught E, Sergott RC, et al. Registry initiated to characterize vision loss associated with vigabatrin therapy. Epilepsy Behav. 2011;22:710–7.
Sergott RC, Foroozan R, Pellock JM, et al. Four-year results from the Sabril® registry yield a low rate of discontinuation secondary to reports of visual deficits. Poster presentation of late-breaking Abstract #3.303 at the 67th Annual Meeting of the American Epilepsy Society, 9 Dec 2013.
Vital and Health Statistics, 2000 CDC Growth Charts for the United States: Methods and Development, Data from the National Health Examination Surveys, Series 11, #246; 2002. http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf. Accessed 23 Jan 2014.
Rhodin MM, Anderson BJ, Peters AM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76.
Rousseau A, Leger F, Le Meur Y, et al. Population pharmacokinetic modeling of oral cyclosporine using NONMEM: comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Ther Drug Monit. 2004;26:23–30.
Schechter PJ. Clinical pharmacology of vigabatrin. Br J Clin Pharmacol. 1989;27:19S–22S.
Wahl EF, Lahdes-Vasama TT, Churchill BM. Estimation of glomerular filtration rate and bladder capacity: the effect of maturation, ageing, gender and size. BJU Int. 2003;91:255–62.
Acknowledgments
The authors acknowledge Susan K. Paulson, PhD, of Pharma Start, LLC, of Northbrook, IL, USA, and Michael A. Nissen, ELS, of Lundbeck LLC, Deerfield, IL, USA, for their assistance in the preparation and revision of this manuscript. This assistance was fully funded by Lundbeck.
Conflict of interest
These analyses were funded by Lundbeck LLC. Dwain Tolbert is a full-time employee of Lundbeck LLC. Mahlaqa Patel and David L. Wesche were full-time employees of Lundbeck LLC at the time this study was conducted. Aziz Karim is a Lundbeck contract employee. Jace C. Nielsen and Kenneth G. Kowalski, all of Ann Arbor Pharmacometrics Group, Inc. (Ann Arbor, Michigan USA), are paid consultants of Lundbeck LLC.
Role of funding source
These analyses were funded by Lundbeck LLC. The Ann Arbor Pharmacometrics Group authors developed the model and conducted the resulting analyses, with input and feedback from the other authors. All authors were responsible for analysis and interpretation of the data. All authors agreed upon the publication of these data, and reviewed and approved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nielsen, J.C., Kowalski, K.G., Karim, A. et al. Population Pharmacokinetics Analysis of Vigabatrin in Adults and Children with Epilepsy and Children with Infantile Spasms. Clin Pharmacokinet 53, 1019–1031 (2014). https://doi.org/10.1007/s40262-014-0172-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0172-z